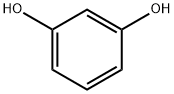
ChemicalBook > CAS DataBase List > Resorcinol
Resorcinol
description Chemical Properties reaction Indications Side effects Precautions Applications Toxicity Uses Production
Resorcinol
- CAS No.108-46-3
- Chemical Name:Resorcinol
- CBNumber:CB5671946
- Molecular Formula:C6H6O2
- Formula Weight:110.11
- MOL File:108-46-3.mol
Resorcinol Property
- Melting point 109-112 °C(lit.)
- Boiling point 281 °C
- Density 1.27
- bulk density 600-700kg/m3
- vapor density 3.8 (vs air)
- vapor pressure 1 mm Hg ( 21.1 °C)
- FEMA 3589 | RESORCINOL
- refractive index 1.5781
- Flash point 340 °F
- storage temp. Store below +30°C.
- solubility H2O: soluble1M at 20°C, clear, faintly yellow
- form Liquid
- pka 9.81(at 25℃)
- Colour Index 76505
- color white
- Odor at 1.00 % in propylene glycol. nutty creamy phenolic hawthorn musty
- PH 4-6 (100g/l, H2O, 20℃)
- explosive limit 1.4%(V)
- Odor Type nutty
- biological source synthetic
- Water Solubility 140 g/100 mL
- Sensitive Light Sensitive
- Merck 14,8155
- JECFA Number 712
- BRN 906905
- Exposure limits TLV-TWA 10 ppm (~45 mg/m3) (ACGIH); STEL 20 ppm (ACGIH).
- Dielectric constant 3.2(0.0℃)
- Stability Stable. Incompatible with strong oxidizing agents. May discolour on exposure to air or light.
- InChIKey GHMLBKRAJCXXBS-UHFFFAOYSA-N
- LogP 0.8 at 20℃
- Substances Added to Food (formerly EAFUS) RESORCINOL
- FDA 21 CFR 177.1210; 310.545
- CAS DataBase Reference 108-46-3(CAS DataBase Reference)
- EWG's Food Scores 6-8
- FDA UNII YUL4LO94HK
- ATC code D10AX02,S01AX06
- NIST Chemistry Reference Resorcinol(108-46-3)
- IARC 3 (Vol. 15, Sup 7, 71) 1999
- EPA Substance Registry System Resorcinol (108-46-3)
- UNSPSC Code 41116107
- NACRES NA.31
Safety
- Hazard Codes :Xn,N
- Risk Statements :22-36/38-50-50/53-41-38
- Safety Statements :26-61-39
- RIDADR :UN 2876 6.1/PG 3
- OEB :A
- OEL :TWA: 10 ppm (45 mg/m3), STEL: 20 ppm (90 mg/m3)
- WGK Germany :2
- RTECS :VG9625000
- F :3-8-9-23
- Autoignition Temperature :605 °C
- TSCA :Yes
- HS Code :2907 21 00
- HazardClass :6.1
- PackingGroup :III
- Hazardous Substances Data :108-46-3(Hazardous Substances Data)
- Toxicity :LD50 orally in Rabbit: 301 mg/kg LD50 dermal Rabbit 3360 mg/kg
-
NFPA 704:
1 3 0
-
Symbol(GHS)




- Signal wordDanger
- Hazard statements H302-H315-H317-H318-H370-H371-H410
- Precautionary statements P273-P280-P301+P312-P302+P352-P305+P351+P338-P308+P311
Resorcinol Price
More Price(36)
- Brand: Sigma-Aldrich(India)
- Product number: W358908
- Product name : Resorcinol
- Purity: ≥98%, FG
- Packaging: 1SAMPLE
- Price: ₹5141.88
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W358908
- Product name : Resorcinol
- Purity: ≥98%, FG
- Packaging: 1KG
- Price: ₹7447.6
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W358908
- Product name : Resorcinol
- Purity: ≥98%, FG
- Packaging: 5KG
- Price: ₹21920.63
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: W358908
- Product name : Resorcinol
- Purity: ≥98%, FG
- Packaging: 10KG
- Price: ₹34943.1
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: R5645
- Product name : Resorcinol
- Purity: BioXtra, ≥99%
- Packaging: 100G
- Price: ₹5412.5
- Updated: 2022/06/14
- Buy: Buy
Resorcinol Chemical Properties,Usage,Production
-
description
Resorcinol has bactericidal, fungicidal and anti-itching effect with the bactericidal effect being 1/3 of the phenol and also a low irritating and corrosive property. At low concentration, it can play the role promoting the regeneration of horny while having keratin exfoliation effect at high concentration. It is mainly used for rubber adhesives, plastics, synthetic resins, synthetic fibers, dyes, preservatives, anti-itch, anti-fungal agent, analytical reagent and can also be used to treat ringworm, eczema, seborrheic dermatitis, acne and psoriasis.
Figure 1 3D structure of resorcinol.
The above information is edited by the Chemicalbook of Dai Xiongfeng. - Chemical Properties It is white needle-like crystal. It will become pink upon exposure to light and air or contact with iron. It has a sweet taste. It is soluble in water, ethanol, amyl alcohol, easily soluble in ether, glycerol, slightly soluble in chloroform, carbon disulfide as well as in benzene.
-
reaction
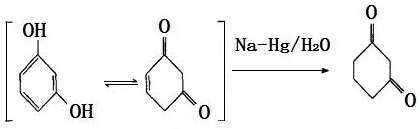
The chemical property of resorcinol is active and it can participate in the following four kinds of reaction.
(1) It can have reaction with sodium amalgam, water for production of dihydro-resorcinol (1, 3-cyclohexanedione).
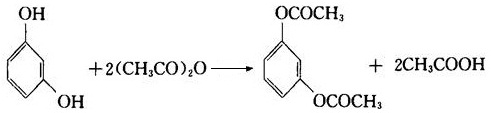
(2) It can generate ester with reaction with acid anhydride.
(3) It can react with hydroxylamine in a diketone type to generate oxime.
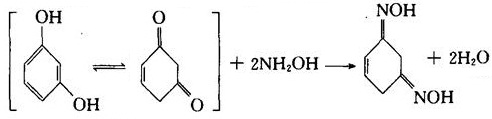
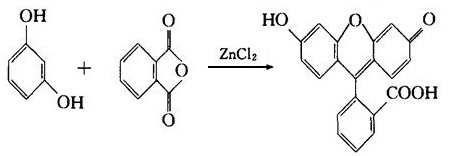
(4) In the action of concentrated sulfuric acid or zinc chloride, it can react with phthalic anhydride to generate fluorescent dye-fluorescent yellow.
- Indications It can be used for the treatment of seborrheic dermatitis, acne, superficial skin fungal infections, tinea versicolor, calluses, corns, and common warts.
-
Side effects
1, it can cause contact dermatitis and has weak irritation effect on skin and mucous membrane. This product, when being absorbed through broken skin and wound surface in the large amount, can lead to myxedema.
2, because this product can be absorbed through the skin or ulcers, and thus is not suitable or being applied to infants and young children in high concentration and large-scale.
3, poisoning symptoms include diarrhea, nausea, vomiting, stomach pain, dizziness, severe or persistent headache, fatigue or weakness, being prone to excitement or irritability, sleepiness, sweating, bradycardia, and shortness of breath.
4, applying this product to the wound of children can lead to methaemoglobinaemia.
5, since this product has anti-thyroid effects, systemic effects is similar as phenol poisoning, but often accompanied with convulsions. -
Precautions
1, it can turn pale hair to black color; it can cause skin redness and scaling at a few days after treatment; take this drug with caution.
2, dark-skinned patients may be caused by its stimulation of pigment generation.
3, when being used in combination with soaps, cleansers, acne preparations, preparations containing alcohol or acid-dimensional A, it can cause skin irritation or excessive drying effect.
4, it has anti-thyroid effects; it can lead to myxedema upon long term use (especially used in ulcer surface).
5. This product is toxic and thus this product can’t subject to systemic or long-term use; avoid applying it to the broken skin wound in order to preventing poisoning. - Applications Resorcinol is an important raw material for organic synthesis. It is mainly used as the raw material of rubber adhesives, raw analysis reagents, drugs and preservatives, dyes, synthetic resin. For example, eosin is an important triphenylmethane dye with red dying which is mainly used for yarn dyeing. It is made from the following process: first generate its intermediate fluorescent yellow thorough the co-heating between resorcinol and phthalic anhydride in the presence of zinc chloride or concentrated sulfuric acid, then apply tetra-bromination to generate it. Eosin is commonly used to produce red ink, also be used as biological material such as the staining agent for microscopic examination. If the intermediate fluorescent yellow is subject to bromination in acetic acid solution, only di-bromination can occur; further co-heating with mercuric acetate can obtain mercurochrome (also known as red mercury bromine) which is an important antiseptic disinfectant. It is easily soluble in water with its 2% aqueous solution being the daily-used “red syrup” for disinfection. The alcohol and acetone solution of mercurochrome may also be used for skin disinfection. Put resorcinol and hexanoic acid for acylation reaction and further reduction can generate 4-n-hexyl hydroquinone which is also a kind of disinfectant. In medicine, it is also used as a topical anti-itch agent and a digestive agent of the intestinal tract.
- Toxicity GRAS (FEMA).
-
Uses
1. It can be used as the raw materials for the production of synthetic resins, adhesives, dyes and ultraviolet absorbing agent. It can also be used as the dipped cord of tire. Medically it can be used as the disinfection antiseptic agent.
2. Resorcinol is also known as 1, 3-hydroquinone. In the field of pesticides, it can be used as the intermediate 3-chloro-4-methyl coumarin and herbicide oxyfluorfen in the synthesis of pesticides coumaphos. It can also be used for the production of dyes, specialty coatings, pharmaceuticals, photographic material, synthetic resins, adhesives, and cosmetics.
3. Resorcinol is mainly used for the field of rubber adhesives, synthetic resins, dyes, preservative, pharmaceutical and analytical reagents. Resorcinol is similar with phenol and cresol. It can generate condensation polymer through the reaction with formaldehyde. It can be used for making glue silk and the adhesive agent of tire cord for nylon-purpose, making wood glue, and being as the adhesive for vinyl material and metal. Resorcinol is the intermediate of many kinds of azo dyes and fur dyes as well as the raw material of pharmaceutical intermediates, p-nitrogen salicylic acid. Resorcinol has bactericidal effects and can be used as preservatives for being supplied to cosmetics and dermatological drugs pastes and ointments. The resorcinol derivatives, β-methylumbelliferone can be used as the intermediate for optical bleach; tri-nitro resorcinol is detonator. There is also a considerable amount of resorcinol being used in the production of benzophenone-class ultraviolet absorbers. This product can irritate the skin and mucous membranes, can cause poisoning disease through the rapid absorption by the skin. Minimum lethal dose of rat being subject to subcutaneous injection is 450mg/kg.
4. It can be applied to the fields of photographic film, medicines, dyes and chemical fiber industry.
5. It can also be used as reagents for analysis.
6. It can be used for characterization and determination of zinc, lead, tartaric acid, nitrate and nitrite through colorimetric method; it can also applied to the colorimetric reaction for measuring sugar and Furfuryl alcohol; as the reagent for detecting ketone sugars and lignin; it can also applied to the salt reagent of diazonium compound as well as to organic synthesis. -
Production
1. Benzene sulfonic acid is sulfonated with oleum; further go through neutralization, alkaline melting, acidification, n-butanol extraction, evaporation of the solvent, and distillation to obtain the finished products. 2. It can be produced through the hydrogenation of m-dinitrobenzene into m-phenylenediamine which is further subject to hydrolysis to get the finished product. 3. It can be produced from the hydrolysis of m-aminophenol. Resorcinol can also derived from benzene and propylene using peroxide di-isopropylbenzene method with the process being similar as isopropylbenzene production.
Put benzene, 65% fuming sulfuric acid and sodium sulfate separately into the reactor; control the reaction temperature at 75 ℃ to obtain the sulphonate. Then add anhydrous sodium sulfate to this sulphonate, stir and heat to 175 ℃ for dissolving it; Add sulfur trioxide at this temperature and continue the reaction for an additional 1.5h to generate di-sulphonate (with the content of benzene disulfonic acid being 75%). Use dilute alkali to neutralize the di-sulphonate and remove the excess amount of sulfate salt; the resulting sodium benzene di-sulfonate is gradually added into the molten sodium hydroxide 290 °C; raise the temperature to 325 °C within 15min and further dissolve the alkali melting substance in water; acidify it with sulfuric acid and extract with ether; evaporate the solvent to obtain the finished resorcinol products. - Description Resorcinol is a white crystalline solid with acharacteristic odor and a sweetish taste. Turns pink onexposure to air or light, or contact with iron. Molecularweight =110.12; Boiling point =277.2℃; Freezing/Melting point =108.9℃; Vapor pressure= 0.0002 mmHg at 25℃; Flash point = 127.2℃ (cc); Autoignitiontemperature = 607℃. Explosive limits: LEL = 1.4% at200℃. UEL—unknown. Hazard Identification (based onNFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 0. Highly soluble in water; solubility = 110%.
- Chemical Properties Resorcinol is a white crystalline solid with a characteristic odor and a sweetish taste. Turns pink on expo- sure to air or light, or contact with iron.
- Occurrence Reported found in roasted barley, cane molasses, beer, red wine, white wine, special wine and coffee.
- Uses An aromatic alcohol used as a chemical intermediate
- Uses anthelmintic
- Uses A benzene derivative used as keratolytic and antiseborrheic. Also used in veterinary medicine as a topical antipruritic and antiseptic (has been used as intestinal antiseptic).
- Uses Resorcinol is used in the manufacture of resorcinol–formaldehyde resins, resin adhesives, dyes, drugs, andexplosives; in tanning; in cosmetics; and in dyeing and printing textiles.
- Uses In very mild solutions, resorcinol is used as an anti-septic and soothing preparation for itchy skin. In slightly higher concentrations, resorcinol removes the top layer of the stratum corneum and is used particularly in cases of acne. In still higher concentrations, it can act as an aggressive surface skin exfoliant. Resorcinol can also be used as a preservative. While it is a beneficial skin care ingredient when used in low concentrations, it causes irritation in higher concentrations with a strong burning sensation and a reddening of the skin. used in high concentrations as a peel, resorcinol may cause a variety of problems, including swelling. It is is obtained from various resins.
- Indications Resorcinol (resorcin), a phenol derivative, is less keratolytic than salicylic acid. This drug is an irritant and sensitizer and reported to be both bactericidal and fungicidal. Solutions containing 1% to 2% have been used in preparations for seborrhea, acne, and psoriasis.
- Definition ChEBI: Resorcinol is a benzenediol that is benzene dihydroxylated at positions 1 and 3. It has a role as an erythropoietin inhibitor and a sensitiser. It is a benzenediol, a member of resorcinols and a phenolic donor.
- Aroma threshold values Detection: 6 to 40 ppm
- Synthesis Reference(s) Tetrahedron Letters, 35, p. 8727, 1994 DOI: 10.1016/S0040-4039(00)78482-6
- General Description Very white crystalline solid that becomes pink on exposure to light if not completely pure. Burns although ignition is difficult. Density approximately 1.28 g / cm3. Irritating to skin and eyes. Toxic by skin absorption. Used to make plastics and pharmaceuticals.
- Air & Water Reactions Hygroscopic. Soluble in water.
- Reactivity Profile Resorcine is a weak organic acid. Incompatible with acetanilide, albumin, alkalis, antipyrine, camphor, iron salts, menthol, spirit nitrous ether, and urethane. Can react with oxidizing materials . Has a potentially explosive reaction with concentrated nitric acid [Lewis]. Turns pink on contact with iron.
- Hazard Irritant to skin and eyes. Questionable car- cinogen.
- Health Hazard Inhalation of vapors or dust causes irritation of respiratory tract. Ingestion causes burns of mucous membranes, severe diarrhea, pallor, sweating, weakness, headache, dizziness, tinnitus, shock, and severe convulsions; may also cause siderosis of the spleen and tubular injury to the kidney. Contact with eyes causes irritation. Can be absorbed from wounds or through unbroken skin, producing severe dermatitis, methemoglobinemia, cyanosis, convulsions, tachycardia, dyspnea, and death.
-
Health Hazard
The acute oral toxicity of resorcinol is moderate in most test animals. It is less toxic than phenol or catechol. Ingestion or skin absorption can cause methemoglobinemia, cyanosis, and convulsions. Vapors or dusts are irritant to mucous membranes. Contact with the skin or eyes can cause strong irritation. An amount of 100 mg caused severe irritation in rabbit eyes.
LD50 value, oral (rats): 301 mg/kg (NIOSH 1986). - Fire Hazard Behavior in Fire: Containers may explode.
- Contact allergens Resorcinol is used in hairdressing as a modifier (or a coupler) of the PPD group of dyes. It is the least frequent sensitizer in hairdressers. It is also used in resins, in skin treatment mixtures, and for tanning. Severe cases of dermatitis due to resorcinol contained in wart preparations have been reported.
- Biochem/physiol Actions Resorcinol is an aromatic alcohol serves as an antiseptic. It reduces pain from painful nodules in patients suffering from Hidradenitis suppurativa (HS).
- Safety Profile Human poison by ingestion. Experimental poison by ingestion, intraperitoneal, parenteral, and subcutaneous routes. Moderately toxic experimentally by skin contact and intravenous routes. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. A skin and severe eye irritant. It can cause systemic poisoning by acting as both a blood and nerve poison. In a suitable solvent, this material can readily be absorbed through human skin and can cause local hyperemia, itching, dermatitis, edema, and corrosion associated with enlargement of regonal lymph glands as well as serious systemic disorders such as restlessness, methemoglobinemia, cyanosis, convulsions, tachycardia, dyspnea, and death. These same symptoms can be induced by ingestion of the material. For poisoning, treat symptomatically. Get medical advice. Used as a topical antiseptic and keratolytic agent. Combustible when exposed to heat or flame; can react with oxidming materials. To fight fEe, use water, CO2, dry chemical. Potentially explosive reaction with concentrated nitric acid. Incompatible with acetadde, alkalies, ferric salts, spirit nitrous ether, urethan. When heated to decomposition it emits acrid smoke and irritating fumes
- Potential Exposure Resorcinol is weakly antiseptic; resorcinol compounds are used in the production of resorcinol-formaldehyde adhesives; or as an intermediate; in pharmaceuticals and hair dyes for human use. Major industrial uses are as adhesives in rubber products and tires, wood adhesive resins, and as ultraviolet absorbers in polyolefin plastics. Resorcinol is also a by-product of coal conversion and is a component of cigarette smoke. Thus, substantial opportunity exists for human exposure.
- First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobin in urine. Emergency treatment and management is similar to phenol.
-
Carcinogenicity
Acute Toxicity. The primary signs of intoxication
resemble those induced by phenol, and include initial stimulation
of the CNS, followed by depression, renal glomerular
and tubular degeneration, central hepatic necrosis, myocardial
depression, pruritus, and reddening of the skin. Resorcinol
has been reported to be less toxic than phenol or
pyrocatechol by oral and dermal routes.
Resorcinol is a simple aromatic chemical (1,3-benzenediol) that has found widespread use, particularly as a coupler in hair dyes. Clinical experience clearly shows that resorcinol is a skin sensitizer, although several predictive tests have been negative. In a local lymph node assay performed in accordance with OECD Guideline 429, resorcinol was identified as a skin sensitizer.
Few reports of the toxicity of resorcinol have been published. Oral ingestion in humans may cause methemoglobinemia, cyanosis, and convulsions, whereas dermal exposure has been reported to cause dermatitis, hyperemia, and pruritus. Industrial inhalation exposures are rather rare, but could occur in any industry if the compound is heated beyond 300°F.
Pathology reported for humans includes anemia, marked siderosis of the spleen and marked tubular injury in the kidney, fatty changes of the liver, degenerative changes in the kidney, fatty changes of the heart muscle, moderate enlargement and pigmentation of the spleen, and edema and emphysema of the lungs. - storage Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Before entering confined space where this chemicalmay be present, check to make sure that an explosive concentration does not exist. Store in tightly closed containersin a cool, well-ventilated area away from oxidizers, oil, ferric salts, methanol, acetanilide, albumin, antipyrine, andurethane. Where possible, automatically transfer materialfrom drums or other storage containers to process containers. Sources of ignition, such as smoking and open flames,are prohibited where this chemical is handled, used, orstored. Metal containers involving the transfer of this chemical should be grounded and bonded. Wherever this chemical is used, handled, manufactured, or stored, useexplosion-proof electrical equipment and fittings.
- Shipping UN2876 Resorcinol, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
- Purification Methods Crystallise resorcinol from *benzene, toluene or *benzene/diethyl ether. The benzoate has m 117o. [Beilstein 6 IV 2069.]
- Incompatibilities Reacts with oxidizers, nitric acid; oil, ferric salts; methanol, acetanilide, albumin, antipyrene, alkalies, urethane, ammonia, amino compounds. Hygroscopic; absorbs moisture from the air.
- Toxics Screening Level The ITSL for resorcinol (108-46-3) is 27 μg/m3 based on a 24-hour averaging time.
- Waste Disposal Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern- ing storage, transportation, treatment, and waste disposal. Dissolve in a combustible solvent and incinerate.
Resorcinol Preparation Products And Raw materials
Raw materials
Preparation Products
Global(905)Suppliers
- Argentina 1 Australia 4 Austria 1 Belgium 6 Brazil 2 Bulgaria 2 Canada 4 China 569 Czech Republic 6 Estonia 1 Europe 4 France 6 Germany 21 Hungary 1 India 112 Indonesia 1 Italy 3 Japan 22 Mexico 2 Norway 1 Poland 3 Romania 1 Slovakia 1 South Korea 2 Spain 2 Switzerland 4 Ukraine 1 United Arab Emirates 1 United Kingdom 23 United States 96 Venezuela 1 Global 905
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15531157085;<br/>+8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8804
- Advantage:58
-
Supplier:
Shandong Zhishang New Material Co., Ltd.
- Tel: +8617653113209
- Email:sales002@sdzschem.com
- Country:China
- ProdList:3049
- Advantage:58
-
Supplier:
Shanghai UCHEM Inc.
- Tel:+862156762820<br/>+86-13564624040
- Email:sales@myuchem.com
- Country:China
- ProdList:8032
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Jinan Qinmu Fine Chemical Co.,Ltd.
- Tel: +8618660799346
- Email:sales@qinmuchem.com
- Country:China
- ProdList:1008
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel:+86-15350571055<br/>+86-15350571055
- Email:Sibel@chuanghaibio.com
- Country:China
- ProdList:6085
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5303
- Advantage:58
-
Supplier:
Shaanxi Haibo Biotechnology Co., Ltd
- Tel: +undefined18602966907
- Email:qinhe02@xaltbio.com
- Country:China
- ProdList:997
- Advantage:58
-
Supplier:
Springchem New Material Technology Co.,Limited
- Tel:+86-021-62885108<br/>+8613917661608
- Email:info@spring-chem.com
- Country:China
- ProdList:2068
- Advantage:57
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
Related articles
108-46-3, ResorcinolRelated Search:
- Hydroquinone tert-Butylhydroquinone 4-tert-Butylcatechol Phloroglucinol Isophthalic acid m-Phthalaldehyde m-Phenylenediamine Isophthalic dihydrazide 1,3-Dicyanobenzene Isophthaloyl dichloride 2,4-DIMETHOXYPHENYL ISOCYANATE 3,5-DIMETHOXYBENZOYL CHLORIDE 3,4,5-Trimethoxybenzoyl chloride Colchicine 3,5-Dimethoxybenzonitrile 3,4,5-Trimethoxyphenylacetonitrile 2',3',4'-TRIHYDROXYACETOPHENONE 3,4,5-Trimethoxybenzonitrile
- K00001
- 108-46-3
- Color Former & Related Compounds
- MINTEZOL
- Functional Materials
- Developer
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Aromatics
- Solutions and Reagents
- Research Essentials
- Polyols
- Oxygen Compounds
- Organic Building Blocks
- Inorganic Salts
- Essential Chemicals
- Chemical Synthesis
- Building Blocks
- ACS Grade
- 其他
- 杂质
- 精细化工,有机化学原料
- 现货速发
- 有机化学原料
- 其他危险化学品
- 高端化学
- 现货主打产品
- 酚类高分子材料
- 有机类
- 有机化工原料-酚类
- 大化工产品
- 固体类
- 原料药-人工合成抗感染类药
- 杂质对照品
- 药用辅料
- 分析试剂-显色剂
- 生化试剂
- 标准品和标准物质
- 其他生化试剂
- 通用试剂
- 农业和环境标准品
- 标准品
- 有机产品
- 消杀防腐系列
- 有机合成物
- 危化品
- 有机原料
- 原料药
- 有机化学试剂
- 苯酚类
- 化工原料-1
- 有机化工原料
- 间苯二酚
- 有机化工原料
- 中间体
- 化合物
- 化工助剂
- 化工