ChemicalBook > CAS DataBase List > Piperonyl aldehyde
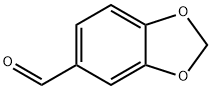
Piperonyl aldehyde
Piperonyl aldehyde
- CAS No.120-57-0
- Chemical Name:Piperonyl aldehyde
- CBNumber:CB3246225
- Molecular Formula:C8H6O3
- Formula Weight:150.13
- MOL File:120-57-0.mol
Piperonyl aldehyde Property
- Melting point 35-39 °C(lit.)
- Boiling point 264 °C(lit.)
- Density 1.2645 (rough estimate)
- vapor pressure 1 mm Hg ( 87 °C)
- refractive index 1.4500 (estimate)
- FEMA 2911 | PIPERONAL
- Flash point >230 °F
- storage temp. Dark Room
- solubility methanol: 0.1 g/mL, clear
- form A crystalline solid
- Odor at 100.00 %. heliotrope flower sweet powdery coconut vanilla
- Odor Type floral
- Water Solubility Slightly soluble
- Sensitive Air & Light Sensitive
- Merck 13,7556
- JECFA Number 896
- BRN 131691
- Stability Stable, but air and light sensitive. Combustible. Incompatible with strong oxidizing agents, bases.
- LogP 1.2 at 35℃
- CAS DataBase Reference 120-57-0(CAS DataBase Reference)
- Substances Added to Food (formerly EAFUS) PIPERONAL
- FDA 21 CFR 182.60
- EWG's Food Scores 1
- FDA UNII KE109YAK00
- NIST Chemistry Reference Piperonal(120-57-0)
- EPA Substance Registry System Piperonal (120-57-0)
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- Hazard Codes :Xi
- Risk Statements :38-52/53
- Safety Statements :61-24/25
- WGK Germany :2
- RTECS :TO1575000
- F :8-10-23
- TSCA :Yes
- HS Code :29329300
- Hazardous Substances Data :120-57-0(Hazardous Substances Data)
- Toxicity :LD50 orally in rats: 2700 mg/kg (Hagan)
-
NFPA 704:
1 0 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H317
- Precautionary statements P261-P272-P280-P302+P352-P333+P313-P362+P364
Piperonyl aldehyde Chemical Properties,Usage,Production
-
Synthesis
Piperonyl alcohol (0.15 g, 1.00 mmol) was dissolved in EtOAc (7 mL, 0.14 M fifinal concentration), and 1-hydroxy-1,2,benziodoxol-3(1H)-one (IBX 0.84 g, 3.00 mmol) was added. The resulting suspension was immersed in an oil bath set to 80 °C and stirred vigorously open to the atmosphere. After 3.25 h (TLC monitoring), the reaction was cooled to room temperature and fifiltered through a medium glass frit. The fifilter cake was washed with 3 × 2 mL of EtOAc, and the combined fifiltrates were concentrated to yield 0.14 g (90%, > 95% pure by 1 H NMR) of piperonal as a waxy solid.
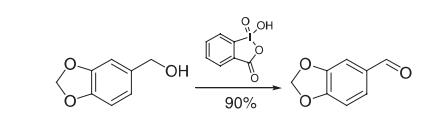
Reference: More, J. D.; Finney, N. S. Org. Lett. 2002, 4, 3001−3003. - Chemical Properties Heliotropin occurs in a number of essential oils, but only in low concentrations. It forms white crystals (mp 37°C) with a sweet, floral, slightly spicy, heliotrope-like odor.
- Chemical Properties Piperonal has a sweet, flowery odor reminiscent of heliotrope and a bittersweet taste.
- Chemical Properties white crystalline solid
- Occurrence Reported found in the essential oils of Robinia pseudo-acacia and Eryngium poterium; in the oils of Spirea ulmaria and of leaves of Doryphora sassafras; also reported found in Tahitian and Bourbon vanilla, camphor wood oil, violet flowers concrete and absolute, burley tobacco, rabbiteye blueberry, melon, pepper, cooked chicken, sherry and dill.
- Uses Piperonal is used as fragrance and flavoring agent.
- Uses In perfumery, in cherry and vanilla flavors, in organic syntheses.
- Uses Piperonal is an impurity of Tadalafil (T004500). Tadalafil impurity A.
- Definition ChEBI: An arenecarbaldehyde that is 1,3-benzodioxole substituted by a formyl substituent at position 5. It has been isolated from Piper nigrum.
-
Preparation
Heliotropin is produced by two main routes:
1) From isosafrole: For many years, oxidative cleavage of isosafrole was the only route applicable on an industrial scale. Isosafrole [120-58-1] is obtained by isomerization from safrole [94-59-7], which can be isolated from (Chinese) sassafras oil . Examples of oxidants that give good yields of heliotropin are chromium(VI) salts, oxygen, and ozone.
This method is still used currently, but the destructive exploitation of sassafras trees in Southeast Asia has led to a strong decline in the availability of sassafras oil and thus of safrole/isosafrole.
2) From catechol: Several routes have recently been developed for the synthesis of heliotropin from catechol. In one such route, catechol is converted into 3,4-dihydroxymandelic acid with glyoxylic acid in an alkaline medium in the presence of aluminum oxide. 3,4-Dihydroxymandelic acid is oxidized to the corresponding keto acid (e.g., with copper-(II) oxide), which is decarboxylated to 3,4-dihydroxybenzaldehyde. The latter product is converted into heliotropin, for example, by reactionwith methylene chloride in the presence of quaternary ammonium salts.
In another route, catechol is first reacted with methylene chloride and converted into 1,2-methylenedioxybenzene . Reaction with glyoxylic acid in strongly acidic media yields 3,4-methylenedioxymandelic acid . Subsequent oxidation and decarboxylation with nitric acid afford heliotropin.
Alternative routes that start from 1,2-methylenedioxybenzene and use piperonyl chloride as intermediate have been described . - Aroma threshold values Detection: 62 ppb to 1 ppm. Aroma characteristics at 1.0%: sweet, anise-like, almond vanilla, floral, black cherry pit, berry raspberry, powdery coumarin-like with a hint of hay.
- Taste threshold values Taste characteristics at 10 to 50 ppm: ripe black cherry fleshy, ripe berry, sweet, macaroon, Jordan almond, creamy vanilla, spicy cream soda, courmarin, slight floral with hay nuances.
-
Synthesis Reference(s)
Canadian Journal of Chemistry, 64, p. 225, 1986 DOI: 10.1139/v86-039
The Journal of Organic Chemistry, 48, p. 4053, 1983 DOI: 10.1021/jo00170a036
Tetrahedron Letters, 33, p. 5909, 1992 DOI: 10.1016/S0040-4039(00)61086-9 - General Description Colorless lustrous crystals.
- Air & Water Reactions Slightly water soluble .
- Reactivity Profile Piperonyl aldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Piperonyl aldehyde is sensitive to light. Piperonyl aldehyde may react with oxidizing materials.
- Fire Hazard Flash point data for Piperonyl aldehyde are not available. Piperonyl aldehyde is probably combustible.
- Flammability and Explosibility Not classified
- Safety Profile Moderately toxic by ingestion and intraperitoneal routes. Can cause central nervous system depression. A human skin irritant. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. See also ALDEHYDES.
- Synthesis By the oxidation of isosafrole with potassium dichromate and sulfuric acid and subsequent steam distillation of piperonal
- Metabolism In the animal body heliotropin undergoes the expected metabolic reaction involving oxidation to the corresponding acid (Williams, 1959).
- Purification Methods Crystallise piperonal from aqueous 70% EtOH or EtOH/water. [Beilstein 19/4 V 225.]
Piperonyl aldehyde Preparation Products And Raw materials
Raw materials
Preparation Products
Global(0)Suppliers
120-57-0, Piperonyl aldehydeRelated Search:
- Paraldehyde Benzaldehyde 4-(Trifluoromethyl)benzaldehyde 2-Methyl-3-(3,4-methylenedioxyphenyl)propanal 4-Dimethylaminobenzaldehyde 4-Hydroxybenzaldehyde alpha-Amylcinnamaldehyde 1,4-Dioxane cyclamen aldehyde p-Tolualdehyde 3-(Trifluoromethyl)benzaldehyde Piperonyl butoxide 2-Nitrobenzaldehyde 1-Nonanal Cuminaldehyde Piperonyloyl chloride 6-Bromopiperonal Piperonyl aldehyde
- Organic Building Blocks
- Chemical Synthesis
- Carbonyl Compounds
- C8
- Building Blocks
- Aldehydes
- Food and Feed Additive
- 食品香料
- 天然等同香料和人造香
- 食用香料(增香剂)
- 医药中间体,有机原料
- 苯并杂环
- 食品添加剂
- 芳香醛
- 有机砌块
- C8
- Carbonyl Compounds
- Building Blocks
- Aldehydes
- Organic Building Blocks
- ALDEHYDE
- CH2O2C6H3CHO
- 他达拉非杂质43(胡椒醛)
- 他达那非杂质43
- 他达拉非杂质43(垂线)
- 他达拉非杂质43
- 胡椒碱,99%
- 洋苿莉醛
- 胡椒醛(洋茉莉醛)
- PIPERONAL 胡椒醛
- 胡椒醛 促肾上腺素
- 一类,胡椒醛,HELIOTROPIN,PIPERONAL
- 洋荣莉醛/胡椒醛
- 3,4-(亚甲二氧基)苯甲醛
- 氧化胡椒醛
- 3,4-亚甲二氧基苯甲醛
- 3,4-亚甲二氧苯甲醛
- 3,4-(亞甲二氧)苯甲醛
- 胡椒醛(0311限出口)
- 3,4-二氧亚甲基苯甲醛
- 亞甲二氧苯甲醛
- 向日[花香]醛
- 向日葵醛
- 3,4-亞甲二氧苯醛
- 兒茶醛基亞甲基醚
- 洋茉莉醛
- 胡椒醛(易制毒)
- 天芥菜醛
- 天芥菜精
- 胡椒甲醛
- 胡椒醛
- 3,4-亚甲基二氧苯甲醛
- 120-57-0
- Tadalafil IMpurity: IMpurity B
- 2H-1,3-BENZODIOXOLE-5-CARBALDEHYDE
- Tadalafil Impurity 43 (Piperonal)
- Heliotroprin
- Benzo[d][1,3]dioxole-5-carbaldehyde