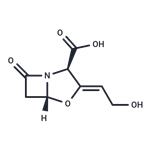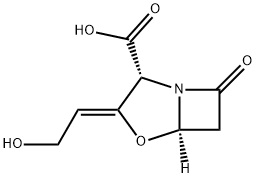
Clavulanic acid
- Product NameClavulanic acid
- CAS58001-44-8
- CBNumberCB9722501
- MFC8H9NO5
- MW199.16
- EINECS261-069-2
- MDL NumberMFCD00867004
- MOL File58001-44-8.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 545.8±50.0 °C(Predicted) |
| Density | 1.65±0.1 g/cm3(Predicted) |
| storage temp. | 4°C, protect from light, stored under nitrogen |
| solubility | H2O : 50 mg/mL (251.05 mM; Need ultrasonic) |
| form | Solid |
| pka | 3.68±0.20(Predicted) |
| color | Off-white to light yellow |
| FDA UNII | 23521W1S24 |
Clavulanic acid Chemical Properties,Usage,Production
Description
Clavulanic acid is a mold product with only weak intrinsic antibacterial activity, but it is an excellent irreversible inhibitor of most β-lactamases. It is believed to acylate the active site serine by mimicking the normal substrate. Hydrolysis occurs with some β-lactamases, but in many cases, subsequent reactions occur that inhibit the enzyme irreversibly. This leads to its classification as a mechanism-based inhibitor (or so-called suicide substrate). The precise chemistry is not well understood, but when clavulanic acid is added to ampicillin and amoxicillin preparations, the potency against β-lactamase–producing strains is markedly enhanced.Originator
Augmentin,Beecham,UK,1981Uses
beta-lactamase inhibitorDefinition
ChEBI: Antibiotic isolated from Streptomyces clavuligerus. It acts as a suicide inhibitor of bacterial beta-lactamase enzymes.Manufacturing Process
100 ml of sterile water was added to a sporing culture which had been grown on Bennetts agar in a Roux bottle for 10 days at 26°C. A mycelium/sporesuspension was produced and used to inoculate 75 liters of steam sterilized medium of the following composition in tap water.
Arkasoy is soybean flour supplied by British Arkady Co., Old Trafford,
Manchester, UK
The pH of the medium was adjusted to 7.0
The medium was contained in a 100 liter stainless steel baffled fermenter, agitated by a 7.5 inch vaned disc impeller at 140 rpm. Sterile air was supplied at 75 liters per minute and the tank incubated for 72 hours at 26°C.
The contents of the seed fermenter were used to inoculate 1,500 liters of steam sterilized medium of the following composition in tap water.
10% Pluronic L81 in soybean oil 0.2% V/V
The pH of the medium was adjusted to 7.0
The medium was contained in a 2,000 liter stainless steel fully baffled fermenter agitated by two 19 inch vaned disc impellers at 106 rpm.
Sterile air was supplied at 1,200 liters per minute. Antifoam was added in 25 ml amounts as required. (10% Pluronic L81 in soybean oil). The fermentation was controlled at 26°C until a maximum yield of clavulanic acid was obtained between 3-5 days when 200-300 μg/ml of clavulanic acid were produced.
Therapeutic Function
AntibacterialWorld Health Organization (WHO)
The amoxicillin/clavulanic acid combination should be reserved for infections likely or known to be caused by amoxicillin- resistant beta-lactamase producing strains. Amoxicillin/clavulanic acid is listed in the WHO Model List of Essential Drugs.Antimicrobial activity
It exhibits broad-spectrum but low intrinsic activity, most MICs being in the range 16–128 mg/L. Enterobacteriaceae and Staph. aureus are among the more sensitive and Ps. aeruginosa the most resistant organisms. MICs of 8 mg/L against H. influenzae and 0.1–4 mg/L for penicillinase-producing N. gonorrhoeae are notable.General Description
Clavulanic acid was found in the culture broth of Streptomyces clavuligerus by Beecham Research Laboratories in 1976. It was the first β-lactamase inhibitor. This antibiotic shows weak antibacterial activity against grampositive and gram-negative organisms but strong inhibitory activity against the β-lactamase produced by ampicillin-resistant bacteria. Clavulanic acid is used orally in combination with amoxicillin and with ticarcillin by injection to enhance the activities of these antibiotics against resistant infections.Pharmacokinetics
It exhibits broad-spectrum but low intrinsic activity, most MICs being in the range 16–128 mg/L. Enterobacteriaceae and Staph. aureus are among the more sensitive and Ps. aeruginosa the most resistant organisms. MICs of 8 mg/L against H. influenzae and 0.1–4 mg/L for penicillinase-producing N. gonorrhoeae are notable.Clinical Use
Clavulanic acid is an antibiotic isolated from Streptomycesclavuligeris. Structurally, it is a 1-oxopenam lacking the6-acylamino side chain of penicillins but possessing a 2-hydroxyethylidene moiety at C-2. Clavulanic acid exhibitsvery weak antibacterial activity, comparable with that of6-APA and, therefore, is not useful as an antibiotic. Itis, however, a potent inhibitor of S. aureus β-lactamaseand plasmid-mediated β-lactamases elaborated by Gramnegativebacilli.Combinations of amoxicillin and the potassium saltof clavulanic acid are available (Augmentin) in variousfixed-dose oral dosage forms intended for the treatment ofskin, respiratory, ear, and urinary tract infections causedby -lactamase–producing bacterial strains. These combinationsare effective against β-lactamase–producingstrains of S. aureus, E. coli, K. pneumoniae, Enterobacter,H. influenzae, Moraxella catarrhalis, and Haemophilusducreyi, which are resistant to amoxicillin alone. The oral bioavailability of amoxicillin and potassium clavulanate issimilar. Clavulanic acid is acid-stable. It cannot undergo penicillanicacid formation because it lacks an amide side chain.Potassium clavulanate and the extended-spectrum penicillinticarcillin have been combined in a fixed-dose, injectableform for the control of serious infections caused byβ-lactamase–producing bacterial strains. This combinationhas been recommended for septicemia, lower respiratory tractinfections, and urinary tract infections caused by β-lactamase–producing Klebsiella spp., E. coli, P. aeruginosa,and other Pseudomonas spp., Citrobacter spp., Enterobacterspp., S. marcescens, and S. aureus. It also is used in bone andjoint infections caused by these organisms. The combinationcontains 3 g of ticarcillin disodium and 100 mg of potassiumclavulanate in a sterile powder for injection (Timentin).
Synthesis
Clavulanic acid is isolated from Streptomyces clavuligerus [60–66], and sulbactam, a sulfone of penicillanic acid, is synthesized from 6-APA [67–69]. Both compounds have extremely weak antibacterial properties and act by forming irreversible complexes with beta-lactamase, which inactivates the enzyme, and as a result the beta-lactam antibiotic has time to destroy the microorganism.Preparation Products And Raw materials
Raw materials
Preparation Products
Clavulanic acid Supplier
Global(97)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 | |
| +8618523575427 | sales@conier.com | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29809 | 58 | |
| +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12825 | 58 | |
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32161 | 58 | |
| +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 22787 | 58 | |
| +86-0571-85134551 | sales@afinechem.com | China | 15352 | 58 | |
| 571-88938639 +8617705817739 |
info@dycnchem.com | China | 52849 | 58 | |
| +8617653113209 | sales002@sdzschem.com | China | 3049 | 58 | |
| +86-852-30606658 | market18@leapchem.com | China | 24727 | 58 |
Related articles
Clavulanic acid is a naturally occurring beta-lactamase inhibitor which was isolated from Streptomyces clavuligerus (Reading and Cole, 1977).
Mar 17,2022
58001-44-8, Clavulanic acidRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine