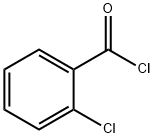
Chemical Properties
clear colourless to very slightly yellow liquid
Uses
2-Chlorobenzoyl chloride, is used in organic synthesis. It is used as a pharmaceutical intermediate for eg as an intermediate of clotrimazolc, preparation of p-chlorobenzoic acid and production of trichloro-acaricide.
Definition
ChEBI: An acyl chloride consisting of benzoyl chloride having a chloro substituent in the ortho-position.
General Description
2-Chlorobenzoyl chloride reacts with aromatic amines and ammonium thiocyanate using polyethylene glycol-400 as the catalyst under the condition of solid-liquid phase-transfer catalysis to form N-aryl-N′(2-chlorobenzoyl) thioureas. 2-Chlorobenzoyl chloride causes the acylation of polystyrene during the preparation and regeneration of the polystyrene-based resin.
Synthesis
A process for preparing 2-chlorobenzoyl chloride which comprises reacting 2-chlorobenzaldehyde with chlorine in the presence of phosphorus pentachloride at a temperature of from 50° to 200° C. and wherein the 2-chlorobenzaldehyde: phosphorus pentachloride ratio is from 1:0.05 to 1:0.2.
Phosphorus pentachloride (41.5 g, 0.2 mole) was added to 2-chlorobenzaldehyde (289.7 g, 2.0 mole, 97%). After the ensuing exotherm had subsided, the resulting red solution was heated to 160° C. A stream of chlorine was introduced through a sparge tube into the solution for six hours. (Gas chromatography indicated 96.5% conversion.) The solution was then cooled and distilled under reduced pressure, giving 325.4 grams of 2-chlorobenzoyl chloride, which boiled at 135° to 140° C. at 16 millimeters of mercury. The yield of 2-Chlorobenzoyl chloride was 93% (98.5% pure).