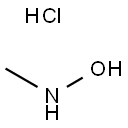
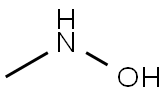
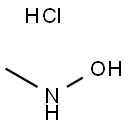
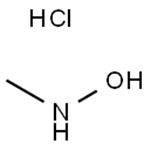
Preparation
N-methylhydroxylamine hydrochloride (N-MHA) was synthesized by a high pressure catalytic hydrogenation method using noble metal Pd/C and Pt-Rh/C as the catalyst. During the synthesis process, the catalyst was easily poisoned and the products would be deeply reduced to methylamine. Therefore, the purity of N-MHA is low and the cost is high. An industrial electrolytic cell was designed for the electrochemical synthesis of N-methylhydroxylamine hydrochloride (N-MHA). Copper was used as the cathode, graphite as the anode, and a cation membrane as the separator. The results show that N-MHA with a high purity of 99% can be electrosynthesized directly from nitromethane in HCl solution.
Reactions
N-methylhydroxylamine hydrochloride was widely used in the 1,3-dipolar cycloaddition and N-methylation reaction. For example, it has been considered an extremely important intermediate in the synthesis of biological active azetidinone and isoxazole compounds such as piperylone, isopyrine, and muscimole.
Synthesis
To a 500 mL three-necked flask equipped with a magnetic stirrer, a thermocouple and an ice water bath was added 224.0 g of crude N-methylhydroxylamine prepared in Example 1. Under the condition of keeping the reaction temperature below 25°C, 110.3 g of 37% aqueous hydrochloric acid solution (10% molar excess) was slowly added dropwise. Upon completion of the reaction, 333.4 g of a mixed methanol/water solution containing N-methylhydroxylamine hydrochloride was obtained.
References
[1] A. Gibson and S. Mirzazadeh. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs.British Journal of Pharmacology.1989, 96 637-644. DOI:
10.1111/j.1476-5381.1989.tb11863.x[2] J. R. Ochoa Gomez. Electrosynthesis of N-methylhydroxylamine.Journal of Applied Electrochemistry.1991, 21 331-334. DOI:
10.1007/BF01020218[3] Reacts with aldehydes and ketones to give nitrones, which undergo 1,3-dipolar addition reactions with alkenes. Cycloaddition to trimethylvinylsilane has been used in a 2-carbon extension of aldehydes to ɑ-unsaturated aldehydes: J. Org. Chem., 49, 3421 (1984). DOI:
10.1021/jo00192a047 [4] Intramolecular cycloaddition has been used as a route to bicyclic systems, e.g. from citronellal: Org. Synth. Coll., 6, 670 (1988).
[5] Reviews: 1,3-Dipolar cycloadditions of nitrones: Synthesis, 205 (1975). The [3+2] nitrone-olefin cycloaddition reaction: Org. React., 36, 1 (1988). Synthetic applications of nitrones: Org. Prep. Proced. Int., 17, 25 (1985).
[6] For conversion to, and reactions of, the N,O-bis(TMS) derivative, see: J. Chem. Soc., Perkin 1, 1823 (1989).
[7] GAN Y, WENKUI Z, HUANG H, et al. Industrial Synthesis of N-Methylhydroxylamine Hydrochloride by Electrochemical Reduction of Nitromethane[J]. Chinese Journal of Chemical Engineering, 2006, 14: 649-653. DOI:
10.1016/S1004-9541(06)60129-8.