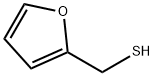
Description
Furfuryl mercaptan has a characteristic unpleasant odor. It is prepared by reacting thiourea and furfuryl chloride with subsequent hydrolysis of the reaction product; also by reduction of difurfuryl disulfide in alcoholic solution using zinc dust and a small amount of acetic acid or using activated alumina. Furfuryl mercaptan tends to polymerize when heated in the presence of mineral acids.
Chemical Properties
2-Furylmethanethiol is an important constituent
of the aroma of roasted coffee. It is a liquid with an unpleasant odor, which
becomes like coffee when diluted.
Chemical Properties
clear colorless to pale yellow liquid
Chemical Properties
Furfuryl mercaptan has a characteristic unpleasant odor.
Occurrence
Reported found in raw and roasted chicken, cooked beef, grilled pork, sesame seed oil, coffee and popcorn; tends to polymerize when heated in the presence of mineral acids
Uses
Furfuryl Mercaptam is a volatile flavor component of corn tortilla chips.
Definition
ChEBI: 2-Furanmethanethiol is a heteroarene.
Preparation
Furfuryl mercaptan is prepared from furfuryl alcohol, thiourea, and hydrogen
chloride.The resulting S-furfurylisothiouronium chloride is cleaved with sodium
hydroxide to give furfuryl mercaptan.
Aroma threshold values
Detection: 0.005 to 0.01 ppb; aroma characteristics at 0.01%: intense sulfurous onion impact, lacrimator, slightly skunk-like with a dairy nuance and a hint of savory and coffee-like notes.
Taste threshold values
Taste characteristics at 0.2 to 1 ppb: sulfureous, roasted, onion, garlic and coffee.
General Description
Darkens on storage
Safety Profile
Poison by
intraperitoneal route. Experimental
reproductive effects. Used as a flavoring in
chocolate, fruit, nuts, and coffee. When
heated to decomposition it emits toxic
fumes of SOx. See also MERCAPTANS.
Synthesis
Prepared by reacting thiourea and furfuryl chloride with subsequent hydrolysis of the reaction product; also by reduction of difurfuryl disulfde in alcoholic solution using zinc dust and a small amount of acetic acid or using activated alumina.