Synthesis
The general procedure for the synthesis of 6-chloropyrimidin-4(3H)-ones from 4,6-dichloropyrimidines is as follows:
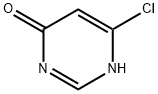
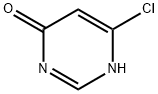
Intermediate 1: Synthesis of 6-chloro-3H-pyrimidin-4-one
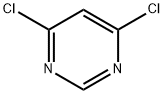
4,6-Dichloropyrimidine (Aldrich; 10.00 g, 67.1 mmol), concentrated hydrochloric acid (50 mL), water (50 mL), and dioxane (50 mL) were mixed and heated at 70 °C for 6 h, and subsequently cooled to room temperature to obtain a pink solution. The solvent was removed by evaporation under reduced pressure (using a vacuum pump) to obtain a pink solid. Ethanol (50 mL) was added to the solid and the mixture was heated until the solid was completely dissolved. The solution was cooled slowly in a warm water bath at about 50°C. After standing overnight, it was filtered to obtain an off-white solid, 6-chloro-3H-pyrimidin-4-one (5.02 g, 57% yield), with a melting point of 193-194°C (literature value: 192-193°C, see DJ Brown and JS Harper, J. Chem. Soc. 1961, 1298-1303).
1H NMR (d6-DMSO) data: δ 6.50 (s, 1H), 8.19 (s, 1H), 13.00 (br s, 1H).
References
[1] Journal of the Chinese Chemical Society, 2013, vol. 60, # 1, p. 27 - 34
[2] Patent: US2009/149466, 2009, A1
[3] Patent: WO2017/59080, 2017, A1. Location in patent: Page/Page column 288; 289