Description
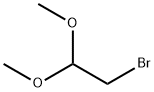
2-Bromo-1,1-dimethoxyethane (Bromoacetaldehyde dimethyl acetal) is used in the synthesis of 2,3-O-acetal via reaction with 2,3-diol in the presence of 10-camphorsulphonic acid (CSA). 2-bromo-1,1-dimethoxyethane was used to react with 4-hydroxylbenzaldehyde, and finally aldehyde substituted prodrug was generated by TFA-mediated deacetalization. It is widely used in chemistry for the synthesis of a variety of antibiotics (including erythromycin and cephalosporins) and other drugs. It is an important and highly reactive bifunctional compound with a good leaving group and a masked aldehyde function. It can be used as a starting material in a variety of reactions to provide N-alkylated compounds, lactams, aldehydes, oximes, azides, and acyclic di-/polyselenides.
Reference
Gotkowska, Joanna. "Bromoacetaldehyde Diethyl Acetal." Synlett26.15(2015):2185-2186.
Chemical Properties
clear colourless to light yellow liquid
Uses
2-Bromo-1,1-dimethoxyethane (Bromoacetaldehyde dimethyl acetal) was used in the synthesis of 2,3-
O-acetal via reaction with 2,3-diol in the presence of 10-camphorsulphonic acid (CSA).
Uses
2-Bromo-1,1-dimethoxyethane (Bromoacetaldehyde dimethyl acetal) was used in the synthesis of 2,3-O-acetal via reaction with 2,3-diol in the presence of 10-camphorsulphonic acid (CSA). 2-bromo-1,1-dimethoxyethane was used to react with 4-hydroxylbenzaldehyde, and finally aldehyde substituted prodrug was generated by TFA-mediated deacetalization.