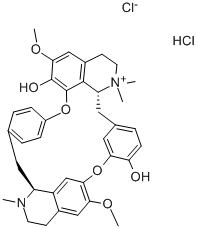
D-TUBOCURARINE CHLORIDE
- Product NameD-TUBOCURARINE CHLORIDE
- CAS57-94-3
- CBNumberCB6296605
- MFC37H41N2O6.ClH.Cl
- MW681.65
- EINECS200-356-9
- MDL NumberMFCD00013471
- MOL File57-94-3.mol
Chemical Properties
| Melting point | 274~275℃ |
| alpha | D20-25 +215° (c = 0.25-0.3 g/100 ml) |
| Density | 1.2074 (rough estimate) |
| refractive index | 1.7350 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble to 25 mM in water and to 10 mM in DMSO |
| form | Powder |
| pka | pK: 7.4(at 25℃) |
| color | white |
| Water Solubility | Soluble to 25 mM in water |
| FDA UNII | 7BCM17J5YA |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
Safety
| Symbol(GHS) |

|
| Signal word | Danger |
| Hazard statements | H300 |
| Precautionary statements | P264-P270-P301+P310-P405-P501 |
| Hazard Codes | T |
| Risk Statements | 25 |
| Safety Statements | 45 |
| RIDADR | UN 1544 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | YO4900000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Toxicity | LD50 in mice, rats (mg/kg): 33.2, 27.8 orally in DMSO; 59.5, 36.9 orally in water (Rosen) |