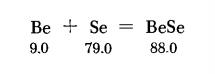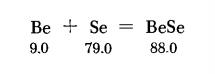Description
Beryllium selenide has the molecular formula of BeSe
and the molecular weight of 87.9722 g/mol. Its CAS
number is 12232-25-6. The literature concerning BeSe is
very sparse and the physical constants such as melting
point have not been published. It can be prepared by
reacting the elements at about 650°C in an inert
atmosphere:
Be+ Se ? BeSe
Beryllium selenide crystallizes in the zincblende unit
cell with a lattice parameter of 5.1521? . Space
group=F43m-Td
2 . It is not very stable in air and tends
to hydrolyze with moisture to form H2Se gas (which
is toxic to humans). Because of this instability, BeSe
has not been employed by itself as a semiconductor
even though its band gap is classified as wide.
Interest in BeSe originates from the fact that formation
of ternary BeZnSe or BeMgZnSe compositions allows
the formation of deposited layers that can be matched
to silicon substrates.
They can also be used to form a blue-green emitting
ZnSe-based laser diode grown on GaSe.
Preparation
Beryllium selenide is prepared from the elements in a Ha stream at 1100°C. Pure Se and pure pulverized Be are placed in a quartz reaction tube in separate boats made of Al2O or BeO, (or at least of quartz). The hydrogen should pass first over the heated Se and then, when laden with its vapor, over the Be. A wash bottle filled with lead acetate is mounted at the exit end of the reaction tube to absorb the very toxic HgSe present in the discharged gas. The Se is heated with a Bunsen burner; the uniform heat of an electric furnace is required for the Be. The BeSe so obtained often shows a tendency to crystallize in long needles.