Description
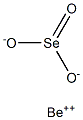
Beryllium selenite has the molecular formula of
BeSeO3 and the molecular weight of 135.969 grams per
mole. It can be prepared by adding sodium selenite to
a solution of beryllium chloride:
BeCl2(aq)+ Na2SeO3(aq) ?(BeOH)2SeO3·2H2O(solid)
It can also be prepared by treating a solution of
beryllium chloride with selenous acid and adding
sodium carbonate to start precipitation. By heating the
dihydrate to 100°C, one obtains the monohydrate,
(BeOH)2SeO3·H2O.