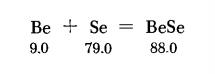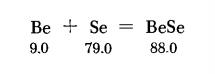Beryllium selenide is prepared from the elements in a Ha stream at 1100°C. Pure Se and pure pulverized Be are placed in a quartz reaction tube in separate boats made of Al2O or BeO, (or at least of quartz). The hydrogen should pass first over the heated Se and then, when laden with its vapor, over the Be. A wash bottle filled with lead acetate is mounted at the exit end of the reaction tube to absorb the very toxic HgSe present in the discharged gas. The Se is heated with a Bunsen burner; the uniform heat of an electric furnace is required for the Be. The BeSe so obtained often shows a tendency to crystallize in long needles.