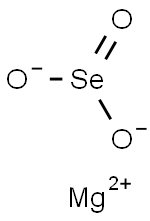Magnesium selenite has the molecular formula of
MgSeO3 and the molecular weight of 151.2658 g/mol.
Its melting point is 557°C. Its CAS number is 15593-
61-0. It is relatively insoluble in water at 0.05454 g/
100 ml. However, the most common form is the hexahydrate
obtained by adding sodium selenite to a solution
of magnesium chloride:
MgCl2(aq)+ Na2SeO3(aq) ?MgSeO3·6H2O(solid)
It can also be prepared by treating a solution of
magnesium chloride with selenous acid and adding
sodium carbonate to start precipitation. The hexahydrate
is a colorless crystal with a density of 2.09 g/cm3
that is insoluble in water. It is soluble in dilute acids. It is orthorhombic in crystal structure and when heated
in a sealed tube to 150°C forms monoclinic prisms of
the dihydrate, when heated to 100°C in air, it loses
5.0 mol of water to form the monohydrate. The monohydrate
then loses the last water of hydration around
190°C to form the anhydrate.
The dihydrate has been the subject of further investigation.
Hydrothermally prepared MgSeO3·2H2O
consists of pairs of edge-sharing MgO6 (4O, 2H2O) octahedra
[dav(Mg–O) = 2.095 ?]. These are linked by pyramidal
SeO3 units [dav(Se–O)=1.697?].
A 7.5 hydrate has also been discovered. The crystal
structure of magnesium selenite as the 7.5-hydrate,
Mg(SeO3)·7.5H2O (space group=P63/mmc), is characterized
by two crystallographically distinct
[Mg(H2O)6]2+
octahedra, one of which is disordered
over two different orientations. The selenite groups
and water molecules (with partially disordered H
atoms) bridge the octahedra via hydrogen bonds.
The selenites, in general, have several versatile and
practical applications. The Mg and Ca salts are used as microfertilizers and repellants in agriculture. They are
also used as catalysts in organic synthesis and they are
precursors for the preparation of pure selenides of stoichiometric
proportion. They are also important intermediate
products in the production and purification of
selenium metal itself.