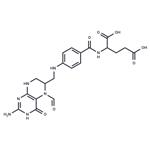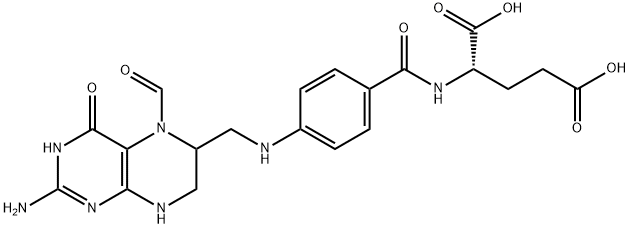
Folinic acid
- Product NameFolinic acid
- CAS58-05-9
- CBNumberCB5681752
- MFC20H23N7O7
- MW473.44
- EINECS200-361-6
- MDL NumberMFCD00867488
- MOL File58-05-9.mol
Chemical Properties
| Melting point | 245°C (rough estimate) |
| Boiling point | 573.92°C (rough estimate) |
| alpha | D20 +14.26° (c = 3.42 as anhydr Ca salt) |
| Density | 1.4485 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | DMSO:172.5(Max Conc. mg/mL);364.35(Max Conc. mM) |
| pka | 3.1, 4.8, 10.4(at 25℃) |
| form | Solid |
| color | Pale Yellow to Light Yellow |
| Water Solubility | >1350g/L(25 ºC) |
| Stability | Hygroscopic |
| CAS DataBase Reference | 58-05-9(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | folinic acid |
| FDA UNII | Q573I9DVLP |
Safety
| Symbol(GHS) |

|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazardous Substances Data | 58-05-9(Hazardous Substances Data) |