Description
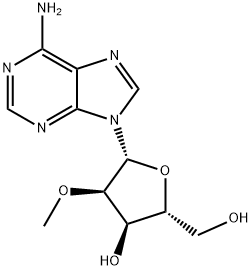
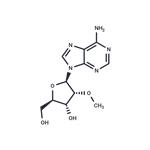
2'-O-Methyladenosine (Cordysinin B) is a member of the class of adenosines that is adenosine in which the hydroxy group at position 2' is replaced by a methoxy group. It has been isolated from the mycelia of Cordyceps sinensis. It has a role as a fungal metabolite. It is a member of adenosines and an ether. It derives from an adenosine. analogue with antiviral properties, starting material for 2'-O'-methyl nucleotides. Potential metabolite of 2'-O-Me-cAMP.
Chemical Properties
White Solid
Uses
2'-O-Methyladenosine is an analog of adenosine used to prepare nucleoside derivatives as inhibitors of RNA-dependent RNA viral polymerase.
Preparation
preparation of 2′-O-Methyladenosine
Methylation of adenosine with methyl iodide in anhydrous alkaline medium
Adenosine is treated with CH3I in an anhydrous alkaline medium at 0°C for 4 h. The major products of this reaction are monomethylated adenosine at either the 2′-O or 3′-O position (total of 64%) and the side products are dimethylated adenosine (2′,3′-O-dimethyladenosi, 21%, and N6-2′-O-dimethyladenosine, 11%). The ratio of 2′-O- and 3′-O-methyladenosine has been found to be 8 to 1. Therefore, this reaction preferentially favors the synthesis of 2′-O-methyladenosine. The monomethylated adenosine is isolated from reaction mixture by a silica gel column chromatography. Then the pure 2′-O-methyladenosine can be separated by crystallization in ethanol from the mixture of 2′-O and 3′-O-methylated isomers. The overall yield of 2′-O-methyladenosine is 42%.
https://doi.org/10.1016/0304-4165(80)90276-7
Definition
2'-O-Methyladenosine is nucleoside modification in which a methyl group is added to the 2' hydroxyl of the ribose moiety of adenosine.
General Description
The 2'-O-Methyladenosine (Am) was showed to be the first nucleotide adjacent to the N
7-methylguanosine (m
7G) cap and it can be further modified at the N
6 position by methylation to generate N6,2’-O-dimethyladenosine (m
6Am) (92% chance of being modified), where the structure of m
7G5'ppp5'm
6AmpNp comprises 20–30% of all the structures[1]. The 2'-O-methyladenosine (Am) modification displaying the lowest level in tumor tissues[2].
Biological Activity
2'-O-Methyladenosine, a methylated adenine residue is found in urine of normals as well as in urine of adenosine deaminase (ADA) deficient patients. 2'-O-Methyladenosine exhibits unique hypotensive activities.
References
[1] Qinghai Li, Guohui Wan, Weiling He. “Methyladenosine Modification in RNAs: Classification and Roles in Gastrointestinal Cancers.” Frontiers in Oncology (2021): 586789.
[2] Qihan He. “Correction: FTSJ1 regulates tRNA 2’-O-methyladenosine modification and suppresses the malignancy of NSCLC via inhibiting DRAM1 expression.” Cell Death & Disease 11 6 (2020): 418.