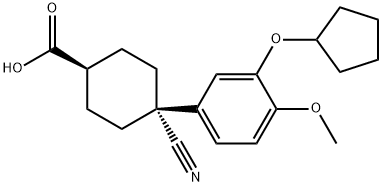
Chemical Properties
Cilomilast is White Solid
Uses
Cilomilast is a selective phosphodiesterase 4 (PDE4) inhibitor and can be used in treatment of chronic obstructive pulmonary disease.
Uses
Selective phosphodiesterase 4 (PDE4) inhibitor. Antiasthmatic; used in treatment of chronic obstructive pulmonary disease.
Definition
ChEBI: 4-cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)-1-cyclohexanecarboxylic acid is a member of methoxybenzenes.
Biological Activity
cilomilast, also known as sb-207499 or ariflo, is a potent second generation inhibitor of type 4 phosphodiesterase (pde4), an enzyme metabolizing cellular cyclic adenosine monophosphate (camp) which acts as a second messenger to disrupt the function of inflammatory cell and induce airway smooth muscle relaxation. cilomilast is currently used for the treatment of chronic obstructive pulmonary disease (copd) due to its strong anti-inflammatory activity as well as inhibitory effects against the release of neutrophil chemoattractants (such as tumor necrosis factor tnf- α, interleukin il-8 and granulocyte-macrophage colony stimulating factor gm-csf) and suppression of the recruitment of neutrophils into tissues and the ltb4 production.m profita, g chiappara, f mirabella, rcdi giorgi, l chimenti, g costanzo, l riccobono, v bellia, j bousquet, and a m vignola. effect of cilomilast (ariflo) on tnf-α, il-8, and gm-csf release by airway cells of patients with copd. thorax 2003; 58: 573-579barry d. zussman, lisa j. benincosa, dawn m webber, david j. clark, hugh cowley, john kelly, robert d. murdoch, james upward, peter wyld, andreas port and hermann fuder. an overview of the pharmacokinetics of cilomilast (ariflo), a new, orally active phosphodiesterase 4 inhibitor, in healthy young and elderly volunteers. j clin pharmacol 2001; 41: 950-958
Biochem/physiol Actions
Cilomilast (SB-207499) is a potent, selective and orally available PDE4 inhibitor that exhibits anti-inflamatory and anti-asthmatic activity.
Mechanism of action
Cilomilast contains the dialkoxyphenyl ring characteristic of selective PDE4 inhibitors. The ether
oxygens hydrogen bond to a glutamine in the binding pocket, and the cyclopentyl ring adds
additional hydrophobic interactions. The oxygen atoms of the carboxyl group form hydrogen
bonds with water that is coordinated with Mg2+ located in the distal end of the binding pocket.
Orally administered cilomilast is 96% bioavailable. Food does not interfere with the overall
absorption; however, food does slow down the rate. Cilomilast is 99% bound to albumin in the
plasma and is metabolized in the liver by CYP2C8. The metabolism is extensive and results in
oxidation, carboxyl group glucuronidation, and dealkylation of the cyclopentyl group, followed by
glucuronidation or sulfation.
Pharmacokinetics
Cilomilast is rapidly absorbed following oral administration (96% bioavailability), has an elimination half-life of 7 hours, and is
extensively metabolized, but not by cytochrom P450 enzymes. The drug shows considerably more selectivity than roflumilast toward
PDE4D, and this might account for the common side effects of nausea and emesis. Studies regarding the benefits of cilomilast
versus placebo in the treatment of asthma have not been very encouraging, but significant improvement was seen in clinical trials of
cilomilast in the treatment of COPD. The side effects of diarrhea and nausea have been considerably higher with cilomilast versus
placebo, but these effects generally are tolerable and self limiting.