Description
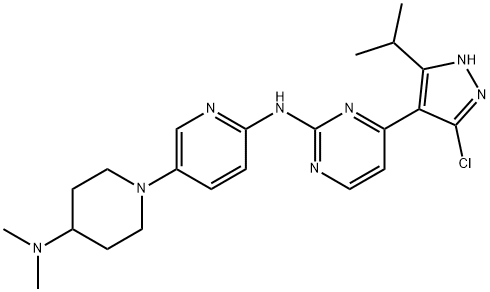
LEE011 (also known as ribociclib) is a CDK4/6 inhibitor. It has been recently approved by FDA (brand name: Kisqali®) to be combined with an aromatase inhibitor for initial endocrine-based therapy for treatment of postmenopausal women with hormone receptor positive, human epidermal growth factor receptor-2 negative (HR+/HER2-) advanced or metastatic breast cancer. It inhibits the growth of tumor cells by arresting the cells at the G1 checkpoint, which prevents the tumor cells from proliferating. In the phase III trial, it demonstrating statistically significant improvement in progression-free survival (PFS) compared to letrozole alone at the first pre-planned interim analysis.
References
S. Kim et al. "Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6- Reactivating Rb in cancer." Molecular Cancer Therapeutics 12.11_Supplement (2013):PR02-PR02.
Bardia, Aditya, et al. "Phase Ib/II study of LEE011, everolimus, and exemestane in postmenopausal women with ER+/HER2-metastatic breast cancer." Asco 2014.
https://www.novartis.com/news/media-releases/novartis-kisqalir-ribociclib-lee011-receives-fda-approval-first-line-treatment