Bioactivity
Alectinib (CH5424802) is an efficient ALK inhibitor. Its IC50 is 1.9nM and it is sensitive to the L1196M mutant, which is highly selective for ALK than PF-02341066, NVP-TAE684 and PHA-E429.
Solubility
In vitro (25 ° C): DMSO-2 mg/mL heating (4.14 mM); Water-<1 mg/mL (<1 mM); Ethanol-<1 mg/mL (<1 mM)
In vivo (25 ° C): 30% PEG 400/0.5% Tween 80/5% propylene glycol-30 mg/mL.
In vitro
Alteinib (CH5424802) can act on ALK and it is ATP-competitive, with a dissociation constant (KD) of 2.4 nM. CH5424802 has a strong inhibitory effect on ALK and L1196M. The Ki is 0.83 and 1.56 nM, respectively. CH5424802 acts on NCI-H2228 NSCLC cells expressing EML4-ALK, inhibiting ALK autophosphorylation. CH5424802 can also inhibit STAT3 and AKT, rather than ERK1/2 phosphorylation. CH5424802 completely inhibited Tyr705, a phosphorylation site of STAT3. CH5424802 preferentially acted on NCI-H2228 cells expressing EML4-ALK without acting on negative NSCLC cell lines that contains ALK, including monolayer cultured HCC827 cells (deletion of EGFR exon19), A549 cells (KRAS mutations) or NCI-H522 cells (EGFR wild type, KRAS wild type, and ALK wild type). CH5424802 acted on NCI-H2228 spherocytes, causing apoptosis-caspase-3/7-like activation. CH5424802 cam inhibit the growth of two lymphoma cells, KARPAS-299 and SR, containing NPM-ALK fusion protein and does not affect the growth of HDLM-2 lymphoma cells without ALK fusion. CH5424802 acted on KARPAS-299 with highly targeted selectivity and stronger antiproliferative activity. When CH5424802 inhibits KAPRAS-299, the IC50 is 3 nM. When inhibit KDR, IC50 was 1.4 μM. The metabolic stability of CH5424802 was high.
In vivo
The oral treatment of CH5424802 can inhibit tumor growth. This role is dose-dependent, and the ED50 is 0.46 mg/kg. CH5424802 was treated at a dose of 20 mg/kg, causing a rapid regression of the tumor, with a recurrent rate of 168%. After 11 days of treatment (at day 28), the tumor volume in each mouse was less than 30 mm3, maintaining an effective anti-tumor effect; no tumor regurgitation occurs in no drug treatment period whitin 4 weeks.The half-life and oral bioavailability for CH5424802 treated mice were 8.6 hours and 70.8%, respectively. The mean plasma levels were 1.7, 1.5, and 0.3 nM after the treatment of 6 mg/kg repeated doses for 2, 7, and 24 hours.CH5424802 treatment inhibited tumor growth.Use CH5424802 to treat KARPAS-299 and NB-1 at a dose of 20 mg/kg, and on day 20, tumor growth was inhibited by 119% and 104%.CH5424802 inhibited STAT3 phosphorylation, which is dose-dependent (2-20 mg/kg), a decrease in phosphorylation of AKT was observed in the xenografts treated with CH5424802.
ALK Inhibitor
Rizotinib is a first-generation anaplastic lymphoma kinase-tyrosine kinase inhibitor (ALK-TKI) and plays an important role inthe treatment of ALK-positive advanced non-small cell lung cancer (NSCLC). However, as with other TKIs, resistance development can-not be avoided with crizotinib. Alectinib is a second-generation ALK-TKI developed by Roche, which can effectively solve the problem of crizotinib resistance.
Binding Mode
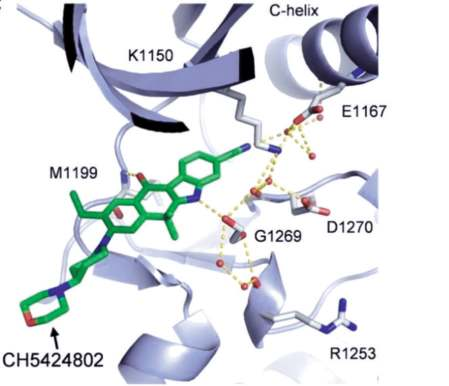
In the co-crystal structure with human ALK (Fig.
2), alectinib binds to the ATP site with the DFG-in
mode, forming one hinge hydrogen bond between
the carbonyl oxygen and the backbone NH of
Met1199 (vs. two or three hinge hydrogen bonds
observed with other ALK inhibitors, such as crizotinib,
lorlatanib, brigatinib). This distinct hinge binding
likely contributes to its high ALK selectivity. The
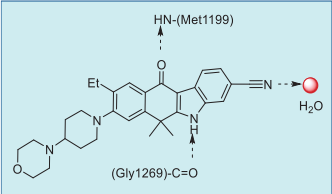
indole NH hydrogen bonds to amide carbonyl of Gly1269, and the cyano group participates in a
complicated network of hydrogen bonds to the
neighboring amino acids via the co-crystallized water
or ethylene glycol molecules (Fig. 3).
Description
Alectinib is another selective ALK inhibitor that is able to overcome resistance
mutations from prior crizotinib exposure [38].
Chemical Properties
White to Off-White Solid
Uses
CH5424802 is a highly selective and potent anaplastic lymphoma kinase (ALK) inhibitor capable of blocking the resistant gatekeeper mutant, which results in reduced cell growth. CH5424802 have been clinically evaluated for the treatment of patients with ALK-driven tumors. Potent ALK inhibitor
Indications
Alectinib (Alecensa(R), Roche) was approved first in Japan in 2014 and then by US FDA in 2015 as a second-generation ALK inhibitor for NSCLC treatment on patients who have progressed or do not tolerate crizotinib. Developed through a structure-based drug design approach, alectinib is a benzocarbazolone derivative that has shown potent inhibitory activity against ALK (IC50 value of 1.9 nM) and gatekeeper mutant L1196M ALK (IC50 value of 1.6 nM). Alectinib is effective with crizotinib-resistant ALK mutations on L1196M, F1174L, R1275Q, and C1156Y. In addition, an array of small-molecule inhibitors are currently being evaluated by several clinical trials for ALK-driven tumors.
Definition
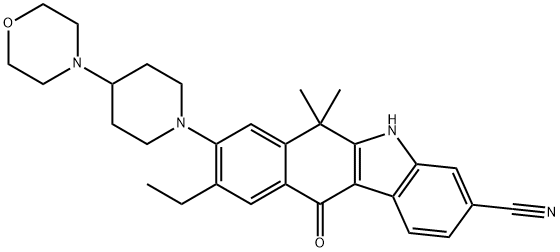
ChEBI: An organic heterotetracyclic compound that is 6,6-dimethyl-5,6-dihydro-11H-benzo[b]carbazol-11-one carrying additional cyano, 4-(morpholin-4-yl)piperidin-1-yl and ethyl substituents at positions 3, 8 and 9 respectively. Used (as the hydr
chloride salt) for the treatment of patients with anaplastic lymphoma kinase-positive, metastatic non-small cell lung cancer.
General Description
Class: receptor tyrosine kinase; Treatment: NSCLC; Oral bioavailability = 37%;
Elimination half-life = 33 h;
Protein binding = >99%
in vitro
in cell free assays the ic50 of ch5424802 for enzyme activity of alk was 1.9 nm; the dissociation constant (kd) value for alk in an atp-competitive manner was 2.4 nm. the inhibitory activity for two hot spot-activating mutations (f1174l and r1275q) in neuroblastoma was comparable to that for wildtype alk [1].
in vivo
in the nci-h2228 model, oral administration of ch5424802 resulted in dose-dependent tumor growth inhibition (ed50 = 0.46 mg/kg) and tumor regression. moreover, treatment of 20 mg/kg ch5424802 showed rapid tumor regression and tumor regrowth did not occur throughout the 4-week drug-free period [2].
target
Primary targets: ALK (IC50 = 1.9 nM)/RET (IC50 = 4.8 nM)
References
1) Sakamoto?et al.?(2011),?CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant; Cancer Cell,?19?679
2) Kodama?et al.?(2014),?Alectinib Shows Potent Antitumor Activity against RET-Rearranged Non-Small Cell Lung Cancer., Mol. Cancer Ther.?13?2910
3) Lu?et al.?(2017),?The second-generation ALK inhibitor alectinib effectively induces apoptosis in human neuroblastoma cells and inhibits tumor growth in a TH-MYCN transgenic neuroblastoma mouse model; Cancer Lett,?400?61