What is Alectinib?
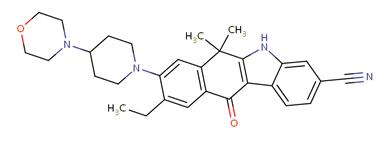
Figure 1. Molecular structure of alectinib
Rizotinib is a first-generation anaplastic lymphoma kinase-tyrosine kinase inhibitor (ALK-TKI) and plays an important role inthe treatment of ALK-positive advanced non-small cell lung cancer (NSCLC). However, as with other TKIs, resistance development can-not be avoided with crizotinib. Alectinib is a second-generation ALK-TKI developed by Roche, which can effectively solve the problem of crizotinib resistance.
Alectinib (trade name Alecensa) is a new type of highly selective ALK-TKI, which was approved by the US FDA on December 11, 2015 for accelerated crocodile patients with advanced (metastatic) NSCLC with ALK gene mutation who have failed or have relapsed due to intolerance after tinib treatment. Alectinib is a benzofluorene carbazole derivative, and its molecular structure is shown in Figure 1. Its molecular structure determines that it can bind well to the kinase region of the ALK gene, thereby ensuring its high selectivity and inhibitory effect on ALK, and its semi-inhibitory concentration (IC50) is 1.9 nM. In vitro studies have found that alectinib can inhibit ALK autophosphorylation and ALK-mediated downstream signalling pathways and phosphorylation of transcriptional activator 3 protein at Tyr705, it can reduce the survival rate of cell lines with ALK protein fusion or activation mutation ability, and its main metabolite M4 has similar activity. In addition, a number of studies have shown that the substrate of alectinib non-P-glycoprotein, a key efflux transporter located in the blood-brain barrier, has better CNS penetration and higher concentration in cerebrospinal fluid, and the inhibitory effect on ALK-positive lung cancer cells is 5 times than that of crizotinib; and it is effective against most acquired drug-resistant mutations after crizotinib treatment, including L1196M, R1275Q, C1156Y, F1174L, etc , But not valid for V1180L, G1202R, I1171T, etc.
About the resistance of alotinib and the countermeasures after resistance:
Ou et al. reported that three patients had G1202R mutation, I1171S mutation and I1171N mutation, respectively, which caused them to be resistant to alectinib. Another study reported that one ALK-positive patient developed resistance to a small cell lung cancer after receiving alectinib. Gainor et al. found that the main drug resistance mechanism of alectinib is caused by known secondary mutations of the ALK tyrosine kinase domain (such as G1202R and I1171T/N/S mutations, etc.), which may affect the binding of alectinib to tumor cells. However, patients with resistance due to mutations in the kinase domain are still largely susceptible to other ALK inhibitors, such as the third-generation 1175ALK TKI Lauratinib (almost all covering the target of alectinib resistance). Even if the patient develops resistance to alectinib, lauratinib can also be chosen to prolong the patient's OS. In addition, Lalatinib is more capable of penetrating the blood-brain barrier. For patients with alectinib resistance, lauratinib will be another option. Furthermore, there are other ALK-TKIs under development, such as entrectinib and ensartinib.
Of course, the choice of follow-up drugs for alectinib resistance is mainly based on the type of resistance mechanism. According to the current research, the third generation of ALK-TKI laratinib can not only overcome the resistance mutations of crizotinib, but also show good efficacy in patients with metastatic disease of the central system in various clinical trials.
A new generation of ALK inhibitors is emerging, providing more and more treatment options for patients with ALK-positive advanced NSCLC. Currently, alectinib (first choice), ceritinib, and crizotinib are recommended first-line for patients with ALK-positive advanced NSCLC. The third-generation drug laratinib may be a "baton" after the treatment failure of first-line ALKTKIs.
References
[1] Iacono D, Chiari R, Metro G, et al. Future options for ALK-positivenon-small cell lung cancer[J]. Lung Cancer,2015,87(3):211-219.
[2] Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity ofthe selective ALK inhibitor alectinib in models of intracranial metas-tases[J]. Cancer Chemother Pharmacol,2014,74(5):1023-1028.
[3] Song Z, Wang M, Zhang A. Alectinib: a novel second generation ana-plastic lymphoma kinase (ALK) inhibitor for overcoming clinically-acquired resistance[J]. Acta Pharm Sin B,2015,5(1):34-37.
[4] Ou SHI, Greenbowe J, Khan ZU, et al. I1171missense mutation (par-ticularly I1171N) is a common resistance mutation in ALK-positiveNSCLC patients who have progressive disease while on alectiniband is sensitive to ceritinib[J]. Lung Cancer,2015,88(2):231-234.
[5] Fujita S, Masago K, Katakami N, et al. Transformation to SCLC afterTreatment with the ALK Inhibitor Alectinib[J]. J Thorac Oncol,2016,11(6):e67-72.
[6] Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resis-tance to first- and second- generation ALK inhibitors in ALK- rear-ranged lung cancer[J]. Cancer Discov,2016,6(10):1118-1133.
[7] https://pubs.acs.org/doi/10.1021/acs.oprd.6b00304
[8] https://pubchem.ncbi.nlm.nih.gov/compound/49806720
You may like
See also
Lastest Price from Alectinib manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 1256580-46-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/Kg/Bag2025-04-21
- CAS:
- 1256580-46-7
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 500kgs
![50-32-8 Benzo[a]pyrene; PAHs; carcinogen; exposure](httpss://www.chemicalbook.com/NewsImg/2019-12-25/20191225918146735.jpg)
