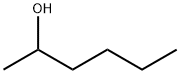
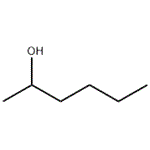
Chemical Properties
colourless liquid
Occurrence
Found among the constituents of several essential oils and aromas, notably apple, strawberry, tea, violet , Java citronella. Bourbon geranium, lavender, lavandin, spike, Litsea zeylanica; also identified in bitter orange . Preparation: By reduction of ethyl caproate with sodium alcoholate or by any other suitable means.
Uses
(+/-)-2-Hexanol is s an important raw material and intermediate used in Organic Synthesis, Pharmaceuticals, Agrochemicals and Dyestuff field. It is used as perfuming agent.
Definition
ChEBI: Hexan-2-ol is a hexanol in which the hydroxy group is at position 2. It has a role as a semiochemical, a plant metabolite and a human metabolite. It is a secondary alcohol and a hexanol.
Synthesis Reference(s)
Journal of the American Chemical Society, 89, p. 1522, 1967
DOI: 10.1021/ja00982a043
Toxicity evaluation
The acute oral LD
50 in mice was reported as 4 g/kg and in rats as 4.87 g/kg and 4.59 g/kg . The acute dermal LD
50 in rabbits was reported as 3.1 ml/kg and as > 5 g/kg . The maximum period of survival of rats inhaling the saturated vapours of hexanol was reported as 8 hr . Gerarde & Ahlstrom found that aspiration of 0-2 ml alcohol C-6 caused respiratory arrest and instant death. Scala & Burtis studied the acute toxicity of commercial grade hexanol by several routes of administration in various species. The acute oral LD
50 in rats was 3.67 g/kg and the acute dermal LD
50 in rabbits was >2.6 g/kg. Dermal application resulted in signs of CNS toxicity. Inhalation exposure of mice, rats and guineapigs to atmospheres nearly saturated with the hexanol preparation for 6 hr elicited a questionable effect on CNS activity, moderate (but reversible) local irritation mainly involving the mucous membranes and slight lung congestion.
General Description
2-Hexanol is one of the constituent of
Porella arboris-vitae extracts which has been determined by solid phase microextraction, gas chromatography-mass spectrometry (SPME GC-MS).
Metabolism
n-Hexanol is metabolized by direct conjugation with glucuronic acid and by oxidation to the carboxylic acid and eventually to CO
2. In the rabbit, direct conjugation is a minor pathway and oxidation the major pathway