Description
Pralatrexate, an injectable DHFR inhibitor, was launched for the treatment of patients with relapsed or refractory PTCL. PTCL is an
aggressive form of non-Hodgkin’s lymphoma (NHL) characterized by
the proliferation of abnormal T-lymphocytes that circulate in the peripheral bloodstream. The inhibition of the folate enzymes DHFR and thymidylate synthase is a well-validated method of cancer treatment.
In vitro, pralatrexate is
slightly less potent than MTX in inhibiting DHFR derived from murine
leukemia L1210 cells (K
i = 18.2 pM vs. 5.75 pM) and human leukemia
CCRF-CEM cells (K
i = 13.4 pM vs. 5.4 pM). However, it is transported
into both types of cells with 10-fold higher efficiency than MTX,
thereby providing a more potent inhibition of cell growth as compared
with MTX. In vivo, intraperitonally administered pralatrexate at 60 mg/
kg twice weekly for three or four doses caused complete lymphoma
regressions in 89, 56, and 30% of HT, RL, and SKI-DLBCL-1 xenografted
mice, respectively, whereas a similar dosing of MTX at 40 mg/kg twice
weekly did not produce complete regression. The posttreatment tumor
diameter was also smaller in pralatrexate-treated animals.
Description
Pralatrexate is a dihydrofolate reductase (DHFR) inhibitor (K
i = 13.4 pM) and antifolate. It inhibits growth of CCRF-CEM acute lymphocytic leukemia cells (IC
50 = 0.04 μM), MDA-468, SK-BR-3, and ZR-75-1 breast cancer cells (IC
50s = 0.11, 0.28, and 0.26 μM, respectively), and SK-LC8 and SK-LC16 non-small cell lung cancer cells (NSCLC; IC
50s = 0.42 and 0.11 μM, respectively).
In vivo, pralatrexate increases median survival from 21 to 40 days when administered in 4 doses of 15 mg/kg over 11 days in an H9 T cell lymphoma mouse xenograft model. Pralatrexate is transported into cells
via the reduced folate carrier (RFC) and undergoes polyglutamation by folylpolyglutamate synthetase (FPGS) to a greater extent than methotrexate or pemetrexed . Formulations containing pralatrexate have been used in the treatment of relapsed or refractory peripheral T cell lymphoma.
Originator
SRI International/ Southern Research Institute/Sloan-Kettering (US)
Uses
An antifolate with high affinity for the reduced folate carrier-type 1, produces marked complete and durable remissions in a diversity of chemotherapy refractory cases of T-cell lymphoma.
Definition
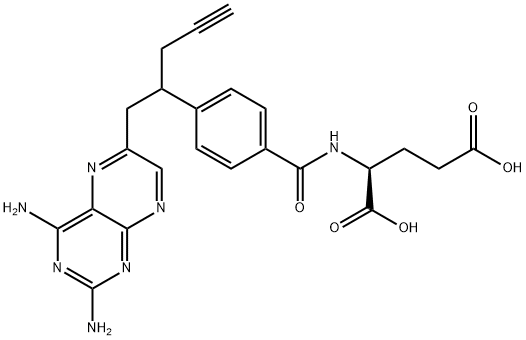
ChEBI: A pteridine that is the N-4-[1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl]benzoyl derivative of L-glutamic acid. Used for treatment of Peripheral T-Cell Lymphoma, an aggressive form of non-Hodgkins lymphoma.
Clinical Use
Pralatrexate, an injectable dihydrofolate reductase (DHFR)
inhibitor, has a superior potency and toxicity profile compared to
other DHFR inhibitors. In 2009, the compound was launched by
Allos and approved in the U.S. for the treatment of patients with
relapsed or refractory peripheral T-cell lymphoma (PTCL) as a single
agent. It is the first drug approved for this indication.70 In 2010,
orphan drug designation was received in the E.U. for the treatment
of cutaneous T-cell lymphoma (CTCL).
Side effects
The most common adverse reactions associated with pralatrexate are mucositis, thrombocytopenia, nausea, and fatigue. Folic acid and vitamin B12 supplements are administered as adjunct therapies to potentially reduce pralatrexate-related hematological toxicity and mucositis.
Synthesis
The chemical synthesis of pralatrexate starts with the alkylation of the anion of dimethyl homoterephthalate with propargyl bromide, promoted by potassium hydride in dimethylformamide, to afford the corresponding a-propargyl diester. Further alkylation of the potassium salt of a-propargyl diester with 2,4-diamino-6-(bromomethyl)pteridine followed by saponification with sodium hydroxide yields a diacid intermediate (2,4diamino- 4-deoxy-10-propargyl-10-deazapteroic acid). Mono-decarboxylation of the diacid intermediate by heating in dimethylsulfoxide at 120 C, followed by coupling with diethyl L-glutamate, and subsequent ester hydrolysis with sodium hydroxide yields pralatrexate.
References
[1]. izbicka e, diaz a, streeper r, et al. distinct mechanistic activity profile of pralatrexate in comparison to other antifolates in in vitro and in vivo models of human cancers. cancer chemother pharmacol, 2009, 64(5): 993-999.
[2]. serova m, bieche i, sablin mp, et al. single agent and combination studies of pralatrexate and molecular correlates of sensitivity. br j cancer, 2011, 104(2): 272-280.