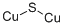Chemical Properties
Copper(I) sulfide, Cu2S, [22205-45-4], MW 159.15, is naturally occurring as the blue or gray mineral chalcocite, [21112-20-9]. Copper(I) sulfide or copper glance is insoluble in water but decomposes in nitric acid and concentrated sulfuric acid.

Copper(I) sulfide is prepared by heating mixtures of copper and sulfur in a hydrogen atmosphere or by precipitation of a copper(II) ammine salt solution with hydrogen sulfide or alkali sulfides. It is used in lubricants, solar cells, semiconductors, and luminous paints.
Physical Properties
Dark-blue or black orthogonal crystals; insoluble in water; slightly soluble in hydrochloric acid; decomposed by nitric acid and concentrated sulfuric acid; moderately soluble in ammonium hydroxide; dissolves in potassium cyanide solutions.
Uses
Copper(I) sulfide is used in luminous paints; antifouling paints; in solidlubricant mixtures; in solar cells; in electrodes; and as a catalyst.
The compound occurs in nature as the mineral chalcocite (copper glance) with varying colors.
Preparation
Copper(I) sulfide is available in nature as the mineral chalcocite. It also may be made by heating copper(II) sulfide with hydrogen, in the presence of small amounts of sulfur.
Alternatively, copper(I) sulfide may be prepared by heating copper with hydrogen sulfide and hydrogen; or by heating the metal with sulfur in an atmosphere of carbon dioxide and methanol vapor.
Reactions
When heated in air, copper(I) sulfide oxidizes forming copper(II) oxide, and sulfur dioxide:
Cu2S + 2O2→ 2CuO + SO2
Heating in the absence of air produces copper(II) sulfide and copper:
Cu2S→ CuS + Cu
When heated with nitric acid, copper(I) sulfide decomposes forming copper nitrate and hydrogen sulfide. The compound dissolves in aqueous solutions containing cyanide ions forming soluble copper-cyanide complexes.
Copper(I) sulfide reacts with polysulfide anions in aqueous solutions forming soluble copper polysulfides.
Chemical Properties
Black powder or lumps.Soluble in nitric acid and
ammonium hydroxide; insoluble in water. Occurs as
the mineral chalcocite.
Uses
Antifouling paints, solar cells, electrodes, solid
lubricants, luminous paints, catalyst.
Uses
Copper(I) sulfide is used in luminous paints, electrodes and in solar cells. It is also used for grain mounts, XRD and in microprobe standards for the identification of unknown minerals. It acts as a precursor for the preparation of copper(I) oxide and copper metal. Further, it acts as a catalyst.
Definition
ChEBI: A copper sulfide in which the metal is in the +1 oxidation state.
Definition
Copper glance: A mineral form ofcopper(I) sulphide,Cu
2S.
General Description
Copper sulfide shows metal-like electrical conductivity, chemical-sensing capability and suitable characteristics for absorption of solar energy. Cu63 NMR, X ray photoelectron spectroscopy,2 copper sulfide CuS was studied in detail. Copper sulfide is a monovalent and oxidation state was determined to be 2.2