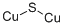Is Cu2S an ionic compound?
Cu2S, also known as copper(I) sulfide, is an ionic compound.
Copper(I) sulfide is also known as Chalcocite. It can be synthesized by the reaction between copper metal and sulfur gas. It is a black color compound not soluble in water.
In chemistry, a "binary copper sulfide" is any binary chemical compound of the elements copper and sulfur. Whatever their source, copper sulfides vary widely in composition with 0.5 ≤ Cu/S ≤ 2, including numerous non-stoichiometric compounds.
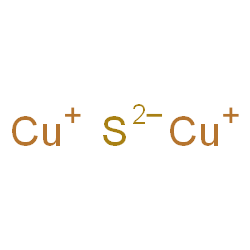
Copper is a transition element, which means it can have different oxidation states. The oxidation state of an ion is its electrical charge. Sulfur is a nonmetallic element that has a consistent oxidation state.
Copper sulfide is a monovalent and oxidation state was determined to be 2.2
Ionic compounds are formed through the transfer of electrons between atoms. In the case of Cu2S, copper (Cu) is a metal, and sulfur (S) is a nonmetal. Metals tend to lose electrons to attain a stable electron configuration, while nonmetals tend to gain electrons.
In the formation of Cu2S, copper loses two electrons to form Cu+ ions, and sulfur gains two electrons to form S2- ions.
The resulting Cu+ and S2- ions are held together by electrostatic forces of attraction due to their opposite charges. This electrostatic attraction between the ions forms the ionic bond. In an ionic compound like Cu2S, the atoms are arranged in a crystal lattice structure.
Therefore, Cu2S is classified as an ionic compound.