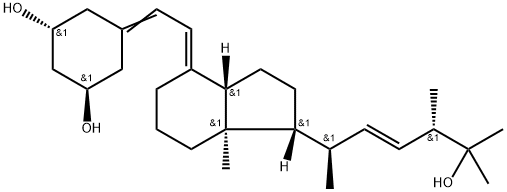
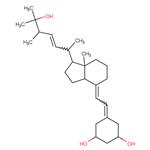
Description
Paricalcitol was launched as Zemplar in the US for the prevention and
treatment of secondary hyperthyroidism associated with chronic renal failure.
Paricalcitol is a synthetic vitamin D2, namely a novel 1-alpha-hydroxy-19-noranalogue
in which the ring A exocyclic methylene group, typical of all vitamin D
systems, has been replaced by two hydrogen atoms. Paricalcitol is the first
vitamin D analogue marketed for this indication. In patients with chronic renal
failure, Paricalcitol appreciably reduced levels of parathyroid hormone (PTH)
without a significant difference in the incidence rate for hypercalcemia or
hyperphosphatemia when compared with placebo.
Description
Paricalcitol is a synthetic 1,25-dihydroxy vitamin D
2 analog. As vitamin D deficiency, associated with chronic kidney disease, leads to an increase in parathyroid hormone (secondary hyperparathyroidism), paricalcitol is used in renal patients to block parathyroid hormone overproduction. Vitamin D deficiency is also a risk factor in cancer, cardiovascular disease, hypertension, and diabetes, and paricalcitol may have applications in those contexts as well.
Chemical Properties
White Solid
Uses
Antihyperparathyriod
Uses
1,25-Dihydroxy vitamin D2 is a potent agonist of the vitamin D receptor. Paricalcitol is a synthetic 1,25-dihydroxy vitamin D2 analog. As vitamin D deficiency, associated with chronic kidney disease, leads to an increase in parathyroid hormone (secondary hyperparathyroidism), paricalcitol is used in renal patients to block parathyroid hormone overproduction. Vitamin D deficiency is also a risk factor in cancer, cardiovascular disease, hypertension, and diabetes, and paricalcitol may have applications in those contexts as well.
Uses
Synthetic analog of vitamin D. Antihyperparathyroid.
Definition
ChEBI: Paricalcitol is a seco-cholestane and a hydroxy seco-steroid. It has a role as an antiparathyroid drug. It is functionally related to a vitamin D2.
brand name
Zemplar (Abbott).
Clinical Use
Vitamin D analogue:
Treatment and prevention of secondary
hyperparathyroidism associated with chronic renal
failure
Metabolism
Extensively metabolised via hepatic and non-hepatic
pathways to form two relatively inactive metabolites.
After oral administration of 3
H-paricalcitol, only about
2% of the dose was eliminated unchanged in the faeces,
and no parent drug found in the urine.
Approximately
70% of the radioactivity was eliminated in the faeces and
18% was recovered in the urine. Most of the systemic
exposure was from the parent drug.
References
[1] ALEX J BROWN. Calcemic activity of 19-Nor-1,25(OH)(2)D(2) decreases with duration of treatment.[J]. Journal of The American Society of Nephrology, 2000, 11 11: 2088-2094. DOI:
10.1681/asn.v11112088[2] JAVIER DONATE-CORREA. Beneficial effects of selective vitamin D receptor activation by paricalcitol in chronic kidney disease.[J]. Current drug targets, 2014, 15 7: 703-709. DOI:
10.2174/1389450115666140417120902[3] MYLES WOLF Ravi T. Vitamin D in patients with renal failure: A summary of observational mortality studies and steps moving forward[J]. Journal of Steroid Biochemistry and Molecular Biology, 2007, 103 3: Pages 487-490. DOI:
10.1016/j.jsbmb.2006.11.009[4] DARKO DUPLANCIC. The influence of selective vitamin D receptor activator paricalcitol on cardiovascular system and cardiorenal protection.[J]. Clinical Interventions in Aging, 2013, 8: 149-156. DOI:
10.2147/cia.s38349