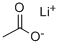Description
Lithium acetate (CH
3COOLi) is a salt of lithium and acetic acid.
Chemical Properties
solid
Uses
Lithium acetate (LiOAc) is a lithium source and a doping salt which can be used in the preparation of plasticized polymer electrolytes for lithium-ion batteries. It can be used as a monovalent cation, which improves the transformation efficiency of
Saccharomyces cerevisiae.
Uses
Lithium acetate is used in the laboratory as buffer for gel electrophoresis of DNA and RNA. It has a lower electrical conductivity and can be run at higher speeds than can gels made from TAE buffer (5-30V/cm as compared to 5 - 10 V / cm). Lithium boric acid or sodium boric acid are usually preferable to lithium acetate or TAE when analyzing smaller fragments of DNA (less than 500 bp) due to the higher resolution of borate-based buffers in this size range as compared to acetate buffers.
Lithium acetate is also used to permeabilize the cell wall of yeast for use in DNA transformation. It is believed that the beneficial effect of LiAc is caused by its chaotropic effect denaturing both DNA, RNA and proteins.
Uses
Lithium acetate hydrate is used in the laboratory as buffer for gel electrophoresis of DNA and RNA. It is also used to permeabilize the cell wall of yeast for use in DNA transformation. It is a metal acetate for proteomics research.
Definition
ChEBI: An acetate salt comprising equal numbers of acetate and lithium ions.
Flammability and Explosibility
Non flammable
reaction suitability
core: lithium
Safety Profile
Poison by intravenous
route. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also ALUMINUM and LITHIUM
COMPOUNDS.