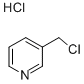
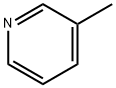
Chemical Properties
White to pale brown solid
Uses
3-(Chloromethyl)pyridine Hydrochloride has been used as a reactant for the synthesis of (E)-3-((1H-Indazol-6-yl)methylene)indolin-2-one derivatives as Polo-like kinase 4 (PLK4) inhibitors.
Application
3-Picolyl chloride hydrochloride is an organic synthetic reagent for the preparation of N,N-bis(pyridin-2-ylmethyl)aniline ligands, 2-oxodihydroquinoline-3-carboxamide derivatives, and 3β-amino-tropane arylamide derivatives with atypical antipsychotic profiles. It is also used in the preparation of novel bifunctional anion exchange resins for nuclear waste treatment.
General Description
Yellow powder or yellow-tan solid with an irritating odor.
Air & Water Reactions
Hygroscopic. Water soluble.
Reactivity Profile
3-Picolyl chloride hydrochloride is incompatible with strong oxidizing agents and strong bases.
Fire Hazard
Flash point data for 3-Picolyl chloride hydrochloride are not available; however, 3-Picolyl chloride hydrochloride is probably combustible.
Safety Profile
Suspected carcinogen
with experimental carcinogenic data. Poison
by ingestion. Mutation data reported. When
heated to decomposition it emits very toxic
fumes of NOx and Cl-.
Synthesis
Chloromethyl pyridine hydrochloride is the important industrial chemicals of various agricultural chemicals, medicine intermediate, Insecticides (tech) & Herbicides (tech), sterilant. A synthetic method of 3-(chloromethyl)pyridine hydrochloride (also known as 3-Picolyl chloride hydrochloride) comprises steps as follows: (1) 3-methylpyridine is taken as a raw material, water is taken as a solvent, and the 3-methylpyridine is oxidized into 3-picolinic acid with potassium permanganate, wherein the molar ratio of the 3-methylpyridine to the potassium permanganate is 1:(2.1-2.3), the oxidizing temperature is kept at 85-90 DEG C, heating is performed for 30 min, a reaction solution is adjusted to be acid after the reaction, and 3-picolinic acid is obtained after cooling and filtration; (2) the 3-picolinic acid and methanol react to produce methyl pyridine-3-carboxylate under the acid condition, and the molar ratio of the 3-picolinic acid to the methanol is 1:1.3; (3) the methyl pyridine-3-carboxylate is reduced to 3-pyridinemethanol; (4) the 3-pyridinemethanol reacts with thionyl chloride to produce a target product, namely, 3-(chloromethyl)pyridine hydrochloride, and the molar ratio of the 3-pyridinemethanol to the thionyl chloride is 1:(1.1-1.3).