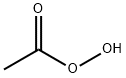
Peracetic
- Product NamePeracetic
- CAS79-21-0
- CBNumberCB00128436
- MFC2H4O3
- MW76.05
- EINECS201-186-8
- MDL NumberMFCD00002128
- MOL File79-21-0.mol
Chemical Properties
| Melting point | -44 °C |
| Boiling point | 105 °C |
| Density | 1.19 g/mL at 20 °C |
| vapor pressure | Low |
| refractive index | n |
| Flash point | 41 °C |
| storage temp. | 2-8°C |
| pka | 8.2(at 25℃) |
| form | liquid |
| color | Colorless liquid |
| Odor | Acrid odor |
| PH | 1 (20°C in H2O) |
| Water Solubility | soluble, >=10 g/100 mL at 19 ºC |
| Merck | 13,7229 |
| BRN | 1098464 |
| Stability | Unstable - may explode on heating. May react violently with organic materials. Incompatible with strong oxidizing agents, acetic anhydride, alkenes, organics. |
| LogP | -0.26 at 20℃ |
| CAS DataBase Reference | 79-21-0(CAS DataBase Reference) |
Safety
| Symbol(GHS) |
   
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H226-H314-H335-H410 | |||||||||
| Precautionary statements | P210-P273-P280-P303+P361+P353-P305+P351+P338+P310 | |||||||||
| Hazard Codes | O,C,N | |||||||||
| Risk Statements | 7-20/21/22-35-50-10-34-22-20 | |||||||||
| Safety Statements | 26-36/37/39-45-61-3/7-23-14A-14-60-9-7-3 | |||||||||
| RIDADR | UN 3109 5.2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | SD8750000 | |||||||||
| F | 4.4-8 | |||||||||
| Autoignition Temperature | Explodes when heated to 110 °C | |||||||||
| HazardClass | 5.2 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29159000 | |||||||||
| Hazardous Substances Data | 79-21-0(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 (mg/kg) in rats: 1540 orally; in rabbits: 1410 dermally; LC50 in rats (mg/m3): 450 by inhalation (Klopotek) | |||||||||
| NFPA 704: |
|