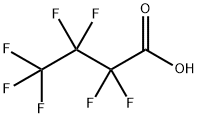
Chemical Properties
Clear colorless to faintly yellow liquid
Uses
Perfluorobutyric acid was used as mobile phase modifier for enhancement of selectivity in the HPLC analysis of histone proteins. It was used as ion pairing reagent in preparative and analytical reserved phase HPLC of underivatised peptides.
Uses
Heptafluorobutyric acid (HBFA) has been used widely for purifying neuropeptides because of its excellent resolution. The use of heptafluorobutyric acid as a volatile ion-pair reagent in the high-performance liquid chromatographic isolation of SB-223070 John K.
Heptafluorobutyric acid is a strong acid and ion-pairing agent that is used in analytical chemistry, notably in HPLC and in gas chromatography/mass spectrometry (GC/MS), in a similar manner to trifluoroacetic acid (TFA). The strong acidity of HFBA ensures that other acidic groups such as carboxylic acid moieties on biomolecules remain protonated, and thus the biomolecule samples are able to interact with organic solvents in such processes as reverse phase chromatography. The longer alkyl chain of HFBA makes it more hydrophobic than TFA, and thus HFBA can be utilized with more hydrophobic samples.
HFBA (0.1%) has been used in the mobile phase of an HPLC/LC-MS protocol for the detection of marine bacterioplankton sideophores.A study of the effects of various acids, including HFBA, on the resolution of intact proteins by reversed-phase LC/ESI-MS has been published.A modified version of the peptide ladder sequencing technique that incorporates allyl isothiocyanate and HFBA has been reported.
Uses
Heptafluorobutyric acid is an ion pair reagent for reverse-phase HPLC. It is used in the sequencing, synthesis, and solubilizing of proteins and peptides. It is used as mobile phase modifier for enhancement of selectivity in the HPLC analysis of histone proteins. It is an effective additive for zinc electrodeposition.
Definition
ChEBI: A monocarboxylic acid that is perfluorinated butyric acid.
General Description
Perfluorobutyric acid is an effective additive for zinc electrodeposition.
Hazard
Irritant to tissue.
Purification Methods
Fractionally distil the acid twice in an Oldershaw column (p 10) with an automatic vapour-dividing head, the first distillation being in the presence of conc H2SO4 as a drying agent. (Take care with the hot acid.) [Beilstein 2 IV 810.]