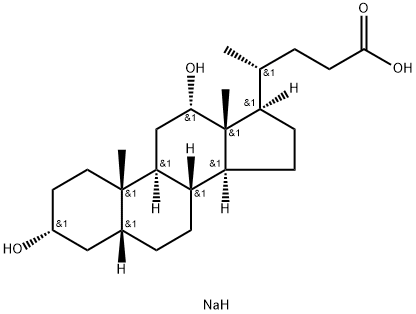
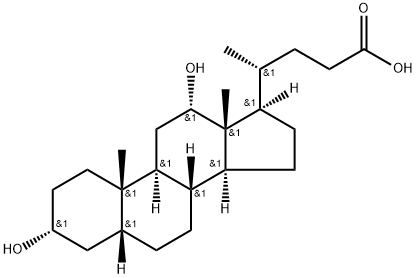
Chemical Properties
Sodium deoxycholate is white to cream crystalline powder
Uses
Sodium deoxycholate is an ionic detergent for the solubilization of membrane-bound proteins.
Uses
Sodium deoxycholate is used as a choleretic and an anionic detergent. It is useful for the extraction of membrane receptors, plasma membrane proteins, nuclei isolation and solubilizes fats as well as membrane components. It acts as an intermediate for the production of corticosteroids and microbiological diagnostic media. In addition, it acts as a catalyst in the preparation of protein extracts for immunoblotting and immunoprecipitation of radiolabeled proteins.
Uses
Sodium deoxycholate is used as a component in cell lysis buffers.
Definition
ChEBI: Sodium deoxycholate is a bile acid salt. It contains a deoxycholate.
General Description
Sodium deoxycholate is a bile salt and an ionic detergent. Bile salts work along with lipids/fats/cholesterol and form mixed micelles in the intestine. These micelles help in fat digestion and absorption through the intestinal wall.
Biological Activity
Sodium deoxycholate (SDC) is an anionic detergent which has been used both to destroy infectious viruses and to fractionate viral components. Sodium deoxycholate begins to bind to the virus at less than 0.1 mM free equilibrium concentration. It causes lysis of the viral membrane at 0.9 +/- 0.1 mM free equilibrium concentration when 2.2 +/- 0.2 - 103 mol of sodium deoxycholate are bound per mol of the virus. The liberation of proteins from the membrane begins at 1.5 +/- 0.1 mM sodium deoxycholate, and the proteins released are virtually free from phospholipids above 2.0 mM sodium deoxycholate. The overall mechanism of sodium deoxycholate solubilization of the viral membrane resembles that of Triton X-100 and sodium dodecyl sulphate, except that with sodium deoxycholate, the various stages of membrane disruption occur at about 10-fold higher equilibrium free detergent concentrations. At sodium deoxycholate concentrations higher than 2.3 mM, the viral spike glycoproteins can be separated by sucrose gradient centrifugation or gel filtration into constituent polypeptides E1, E2 and E3[1-2].
Biochem/physiol Actions
Solubilizes fats for absorption in the intestines.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NasO.
Purification Methods
Recrystallise it from EtOH and dry it in an oven at 100o. The solution is freed from soluble components by repeated extraction with acid-washed charcoal. [Beilstein 10 IV 1608.]
References
[1] A Helenius. “Solubilization of the Semliki Forest virus membrane with sodium deoxycholate.” Biochimica et biophysica acta 436 2 (1976): 319–34.
[2] A E Auletta, M L Marlowe. “Effect of sodium deoxycholate on rubella virus.” Applied microbiology 16 10 (1968): 1624.