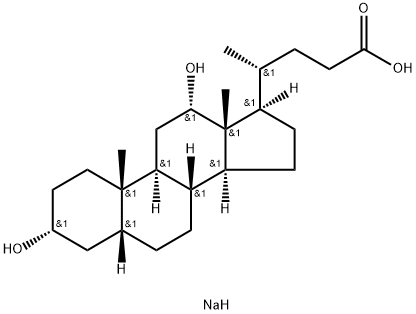
Sodium deoxycholate: synthesis and complex self-assembly behaviour
Sodium deoxycholate (Sodium deoxycholate) is representative of the complex aggregation behaviour of bile salts in aqueous solutions. Small aggregates , thixotropic gel , and different mesophases have been published. Transitions among Sodium deoxycholate structures take place in a narrow pH range, being dependent on ionic strength as well.
Preparation of sodium deoxycholate
Weigh 0.2 g insulin and put to a conical flask for 8 times, add 1g,1.2g,1.4g,1.6g,1.8g,2.0g,2.4g and3.0g sodium deoxycholate respectively, then add 20ml tetrahydrofuran solution containing hydrogen chloride gas (concentration 0.1%, weight/volume, g/ml), stirring for 5 min at room temperature and move to rotary evaporator, remove the solvent by rotary evaporation, at 35 °C, reduced pressure distillation and vacuum drying at room temperature for over 12 hours. 8 groups of complexes powder with weight ratio 1:5∼1:15 of drug/phospholipids are obtained. 8 groups of complexes have no residues of hydrogen chloride gas determined by gas chromatographic method.[1]

The complex self-assembly behaviour of sodium deoxycholate
Bile acids and their salts are produced by mammals and a great number of other animals. They have been widely studied due to their biological and pharmacological activity. Bile salts are rigid steroidal skeleton compounds with two sides, α and β having different polarities, and a flexible isopentanoic side chain. Consequently, bile salts behave as surfactants in aqueous solution forming aggregates above a critical aggregation concentration (cac). Such properties have been largely exploited both in biological and material science applications . Different aggregation models with substantial differences have been published. At low bile salts concentrations, Small proposed the formation of primary micelles where monomers are packed in a back-to-back way. At higher concentrations, larger micelles are formed by primary micelles association. A disk-like aggregate was suggested by Kawamura et al and Vázquez-Tato et al extended this model by including the fraction of counterions. In these models, the hydrophobic faces are oriented towards the inside of the micelle. On the other hand, Giglio et al
proposed a helical packing in which the nonpolar groups were located at the external surface of the helix while the polar parts were placed on the inside.[2]
Israelachvili summarised the most common structures formed by the self-assembly of amphiphilic molecules and how they are determined by the surfactant packing parameter or, equivalently, its hydrophilic-lipophilic balance, HLB. When the HLB is greater than 10, the hydrophobic and hydrophilic parts of the molecule are located at the internal and external region of the aggregate, respectively. Micelles (MI), rods (H) and cubic phases (C) mainly form. Lamellar phases (L) appear at HLB ~ 10. For HLB values lower than 10, monomers orientation in the aggregate is reversed (polar part and water molecules inside the aggregate and outwards, the apolar surface) and the former morphologies emerge, but now inverted (CII-I).
The above described Sodium deoxycholate structures can be placed into this scheme. Small’s and Kawamura’s models agree with the starting micellar-rod stages (MI-H). Giglio and Marques reported structures namely liquid crystals with hexagonal symmetry, spherulitic crystals, fibres and crystals that complete the reverse phases region (I-CII).Due to its pivotal role in physiological processes, the study of bile salts aggregation has been occupying the scientific discussion since the beginning of the previous century. Completely distinct models were proposed for Sodium deoxycholate aggregates by several groups around the world, making their structure determination an open question until now.By showing new phases of Sodium deoxycholate (rods, sponge, vesicles, lamellar sheets, nanotube gel), a more articulated scenario is disclosed: (i) the previous literature and the new results have been rationally linked, (ii) the use of conventional tools for classic surfactant description is extended to not classic surfactant aggregation and (iii) a very rare example of biomolecule, covering alone (without co-assembly molecules) all the self-assembly possibilities for surfactants, is demonstrated.
In the light of Israelachvili's scheme a rational explanation was found for all the proposed Sodium deoxycholate models and observed structures. Even though Sodium deoxycholate is not a conventional (not head-tail) surfactant, the structure transitions were explained referring to an effective HLB change and to a suggested concept that is qualitatively analogue to the packing factor of classic surfactants.On one hand, this reasoning picks up the pieces of the puzzling question about Sodium deoxycholate aggregation models. On the other hand, it highlights an intriguing case: a molecule that, under modulation of the environment condition, covers alone and reversibly the entire spectrum of the assembly possibilities that a classic surfactant would singularly just partially fulfil. We believe that not coincidently such adaptable assembly occurs in a multitasking physiological molecule that drives different functions in spatially (e.g. liver, gallbladder, intestine, blood stream) and chemically (e.g. different pHs, ionic strengths) diverse parts of the body, by coworking with many other biological systems (e.g. proteins, lipids, enzymes, genes, bacteria) .[3]
References
[1] INSTITUTE OF MATERIA MEDICA CAMS - EP2594281, 2013, A1
[2] D'Archivio, A. A., Galantini, L., Giglio, E., & Jover, A. (1998). X-ray and quasi-elastic light-scattering studies of sodium deoxycholate. Langmuir, 14, 4776 - 4781.
[3] Jover, A., Fraga, F., Meijide, F., Vázquez Tato, J., Cautela, J., Del Giudice, A., & di Gregorio, M. C. (2021). Revealing the complex self - assembly behaviour of sodium deoxycholate in aqueous solution. Journal of Colloid and Interface Science, 604, 415 - 428.
You may like
See also
Lastest Price from Sodium deoxycholate manufacturers

US $0.00-0.00/KG2025-09-12
- CAS:
- 302-95-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1MT
US $0.00/kg2025-09-10
- CAS:
- 302-95-4
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200KGS