Description
Ethyl butyrate is an ester soluble in propylene glycol, paraffin oil, and kerosene. It has a fruity odor, similar to pineapple. Ethyl butyrate is present in many fruits e.g. apple, apricot, banana, plum, tangerine etc.
Ethyl butyrate is used as an artificial flavoring resembling orange juice or pineapple in alcoholic beverages, as an ingredient of fragrance, and as a solvent and plasticizer for cellulose. It is also used in the production of polyvinyl butyral (PVB).
Uses
Ethyl Butyrate is used in flavorings, extracts, perfumery, and as a solvent. These are not necessarily FDA approved indications, but instead are uses of liquid nitrogen.It is synthesized by reacting ethanol and butyric acid in a condensation reaction.
Ethyl Butyrate is perceived as a tropical fruit, tutti fruity, mango or pineapple flavour. One of the ester flavours found in most beers, it can also be perceived as a slightly cheesy fruit flavour. Ethyl Butyrate generally occurs in beers due to fermentation and too much is normally considered an off flavour.
Applications: artificial flavoring resembling orange juice or pineapple in alcoholic beverages, as a solvent in perfumes, and as a plasticizer for cellulose.
References
[1] http://www.hmdb.ca
[2] https://en.wikipedia.org/wiki/Ethyl_butyrate
[3] http://www.chemicalland21.com
Description
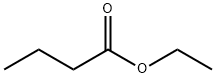
Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH
3CH
2CH
2COO.CH
2CH
3.It is soluble in propylene glycol, paraffin oil, and kerosene.It has a fruity odor, similar to pineapple.
Chemical Properties
Ethyl butyrate is a colorless liquid. Pineapple
odor. The Odor Threshold is 0.015 ppm.
Chemical Properties
Ethyl Butyrate occurs in fruits
and alcoholic beverages, but also in other foods such as cheese. It has a fruity
odor, reminiscent of pineapples. Large amounts are used in perfume and in flavor
compositions.
Chemical Properties
colourless liquid with a fruity odour
Chemical Properties
Ethyl butyrate has a fruity odor with pineapple undertone and sweet, analogous taste.
Occurrence
Identified by gas chromatography in olive oil and other vegetable oils. Reported found in apple, banana, citrus
peel oils and juices, cranberry, blueberry, black currants, guava, grapes, papaya, strawberry, onion, leek, cheeses, chicken, beef, beer,
cognac, rum, whiskies, cider, sherry, grape wines, coffee, honey, soybeans, olives, passion fruit, plums, mushroom, mango, fruit
brandies, kiwifruit, mussels and pawpaw.
Uses
Ethyl butyrate is used in flavors and fragrances. It is used as a solvent in perfumery products, and as a plasticizer for cellulose. It is used in the preparation of novel 2-cyanopyrimidines as cathespin K inhibitors. It is also used in the synthesis of pyridobenzimidazole derivatives exhibiting antifungal activity by the inhibition of β-1,6-glucan.
Uses
manufacture of artificial rum; perfumery; the alcoholic solution constitutes the so-called "pineapple oil".
Uses
It is commonly used as artificial flavoring resembling orange juice or pineapple in alcoholic beverages (e.g. martinis, daiquiris etc.), as a solvent in perfumery products, and as a plasticizer for cellulose. In addition, ethyl butyrate is often also added to orange juice, as most associate its odor with that of fresh orange juice.
Ethyl butyrate is one of the most common chemicals used in flavors and fragrances. It can be used in a variety of flavors: orange (most common), cherry, pineapple, mango, guava, bubblegum, peach, apricot, fig, and plum. In industrial use, it is also one of the cheapest chemicals, which only adds to its popularity.
Definition
ChEBI: Ethyl butyrate is a butyrate ester resulting from the formal condensation of the hydroxy group of ethanol with the carboxy group of butyric acid. It has a role as a plant metabolite. It is functionally related to an ethanol.
Preparation
By esterification of n-butyric acid with ethyl alcohol in the presence of Twichell’s reagent or MgCI2; also by heating
n-butyl alcohol and ethanol over CuO + UO3 catalyst at 270°C
Production Methods
Ethyl Butyrate can be synthesized by reacting ethanol and butyric acid. This is a condensation reaction, meaning water is produced in the reaction as a byproduct.
Aroma threshold values
Detection: 0.1 to 18 ppb
Taste threshold values
Taste characteristics at 20 ppm: fruity, sweet, tutti-frutti, apple, fresh and lifting, ethereal.
General Description
A clear colorless liquid with a pineapple-like odor. Flash point 78°F. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Ethyl butyrate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. May attack some forms of plastics [USCG, 1999].
Hazard
Irritant to eyes and mucous membranes,
narcotic in high concentration. Flammable, dangerous fire risk.
Health Hazard
Inhalation or ingestion causes headache, dizziness, nausea, vomiting, and narcosis. Contact with liquid irritates eyes.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back. Containers may explode in fire.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: May attack some forms of plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Wdly toxic by
ingestion. A skin irritant. Flammable liquid
when exposed to heat or flame; can react
vigorously with oxidizing materials. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also
ESTERS.
Potential Exposure
Ethyl butyrate, and aldehyde, is used
in flavorings, extracts, perfumery, and as a solvent.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Carcinogenicity
Not listed by ACGIH, California
Proposition 65, IARC, NTP, or OSHA.
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Ethyl butyrate is incompatible with oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates), strong acids (such ashydrochloric, sulfuric, and nitric), bases, and heat. Store intightly closed containers in a cool, well-ventilated area.Sources of ignition, such as smoking and open flames, areprohibited where ethyl butyrate is handled, used, or stored.Metal containers involving the transfer of 5 gallons or moreof ethyl butyrate should be grounded and bonded. Drumsmust be equipped with self-closing valves, pressure vacuumbungs, and flame arresters. Use only nonsparking tools andequipment, especially when opening and closing containersof ethyl butyrate.
Shipping
UN1180; UN1178 Ethyl butyrate, Hazard Class: 3;
Labels: 3-Flammable liquid
Purification Methods
Dry the ester with anhydrous CuSO4 and distil it under dry nitrogen. [Beilstein 2 IV 787.]
Incompatibilities
Vapor explosive mixture with air. Ethers
can act as bases. They form salts with strong acids and
addition complexes with Lewis acids. The complex
between diethyl ether and boron trifluoride is an example.
Ethers may react violently with strong oxidizing agents. In
other reactions, which typically involve the breaking of the
carbon-oxygen bond, ethers are relatively inert.
Incompatible with alkaline materials, strong acids, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause
fires or explosions. Keep away from strong bases and heat
Waste Disposal
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal, state,
and local environmental regulations must be observed