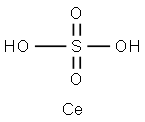
Description
Ceric sulphate salts are yellow to yellow/orange solids that are moderately soluble in water and dilute acids. Its neutral solutions slowly decompose, depositing the light yellow oxide CeO2.
Physical properties
Ceric Sulphate is a white crystalline powder with orthogonal crystal system, insoluble in glacial acetic acid and pure ethanol(96%).
Uses
Ceric sulfate is used in analytical chemistry for redox titration, often together with a redox indicator. It is also used to prepare spherical-like nanoparticles CeO2, nanoparticles for NO reductions' reactions.
Preparation
Ceric Sulphate was historically produced by direct reaction of fine, calcined cerium (IV) oxide and concentrated sulfuric acid, yielding the tetrahydrate.
Health Hazard
Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.