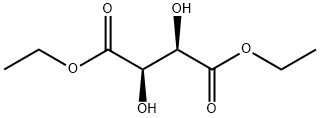
Chemical Properties
Colorless to light yellow liqui
Chemical Properties
Diethyl tartrate has a mild, fruity, wine aroma.
Occurrence
The d-isomer has not been reported found in nature; the l-isomer and the racemic form are of little importance.
Reported found in sherry, white and red wine.
Uses
(+)-Diethyl L-tartrate is used as a chiral auxiliary in the Sharpless enantioselective epoxidation of allylic alcohols, for Sharpless-type enantioselective oxidation of sulfides to sulfoxides and as a chiral auxiliary in the enantioselective construction of cyclopropanes from allylic alcohols by an asymmetric Simmons-Smith reaction. It is also used as a chiral reagent in a host of chemical reactions, such as the synthesis of isoquinoline alkaloids and arundic acid, which has been used in acute ischemic stroke therapy.
Uses
Diethyl L-(+)-Tartrate is used as a chiral reagent in a host of chemical reactions, such as the synthesis of isoquinoline alkaloids and arundic acid, which has been used in acute ischemic stroke thera
py.
Uses
(+)-Diethyl L-tartrate can be used in the synthesis of biologically active compounds such as (+)-altholactone, (-)-aspicilin, (+)-monomorine I and (+)-(1
R,2
R,3
S,6
S)-3,6-di-
O-methyl conduritol-E.
General Description
Made from natural tartaric acid
Flammability and Explosibility
Non flammable