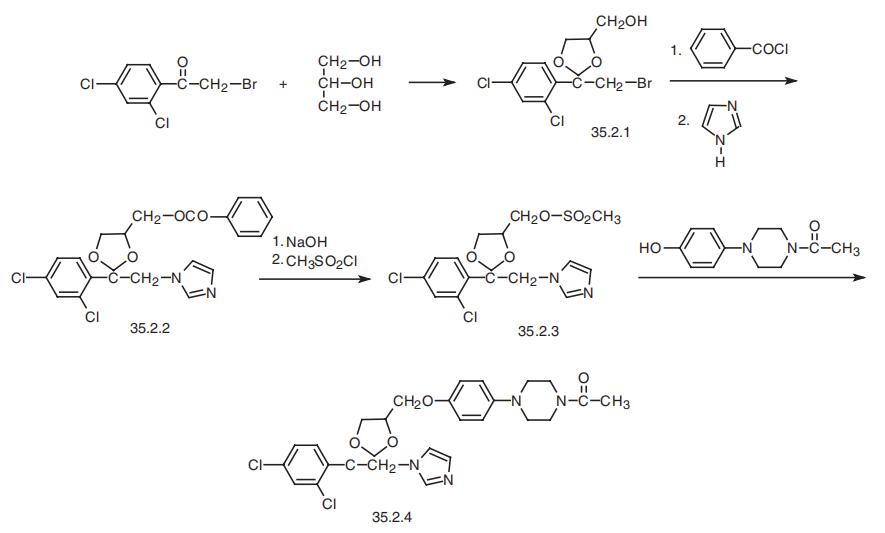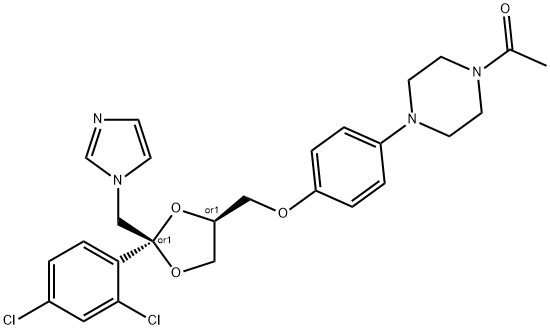
Ketoconazol
Bezeichnung:Ketoconazol
CAS-Nr65277-42-1
Englisch Name:Ketoconazole
CBNumberCB9146879
SummenformelC26H28Cl2N4O4
Molgewicht531.43
MOL-Datei65277-42-1.mol
Synonyma
Ketoconazol
Ketoconazol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 148-152 °C |
| Siedepunkt | 753.4±60.0 °C(Predicted) |
| Dichte | 1.4046 (rough estimate) |
| Brechungsindex | -10.5 ° (C=0.4, CHCl3) |
| Flammpunkt | 9℃ |
| storage temp. | 2-8°C |
| Löslichkeit | methanol: soluble50mg/mL |
| pka | pKa 3.25/6.22(H2O,t =25,I=0.025) (Uncertain) |
| Aggregatzustand | Off-white solid |
| Farbe | white to light yellow |
| Optische Aktivität | [α]20/D -1 to 1°, c = 4 in methanol |
| Wasserlöslichkeit | Soluble in DMSO, ethanol, chloroform, water, and methanol. |
| Merck | 14,5302 |
| BCS Class | 2 |
| Stabilität | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| InChIKey | XMAYWYJOQHXEEK-OZXSUGGESA-N |
| LogP | 4.350 |
| CAS Datenbank | 65277-42-1(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | T,N,F |
| R-Sätze: | 25-36/37/38-23/24/25-50/53-48/22-60-39/23/24/25-11 |
| S-Sätze: | 36-45-36/37/39-26-61-60-53-36/37-16-7 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS-Nr. | TK7912300 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29349990 |
| Giftige Stoffe Daten | 65277-42-1(Hazardous Substances Data) |
| Toxizität | LD50 in mice, rats, guinea pigs, dogs (mg/kg): 44, 86, 28, 49 i.v.; 702, 227, 202, 780 orally (Heel) |