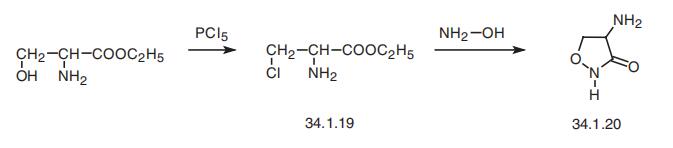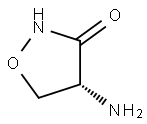
Cycloserin
Bezeichnung:Cycloserin
CAS-Nr68-41-7
Englisch Name:D-Cycloserine
CBNumberCB9138759
SummenformelC3H6N2O2
Molgewicht102.09
MOL-Datei68-41-7.mol
Synonyma
Cycloserin
Cycloserin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 147 °C (dec.)(lit.) |
| alpha | 111 º (C=5, 2N NaOH) |
| Siedepunkt | 191.38°C (rough estimate) |
| Dichte | 1.3516 (rough estimate) |
| Brechungsindex | 1.5110 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| Löslichkeit | water: soluble50mg/mL, clear, colorless to light yellow |
| Aggregatzustand | powder |
| pka | pKa 4.5 (Uncertain) |
| Farbe | white to off-white |
| Optische Aktivität | [α]20/D +115.0±5.0°, c = 2% in H2O |
| Biologische Quelle | synthetic |
| Wasserlöslichkeit | SOLUBLE |
| Sensitive | Air Sensitive |
| Merck | 14,2751 |
| BRN | 80798 |
| BCS Class | 3/1 |
| Stabilität | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20°C for up to 1 month. |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 5-20 |
| S-Sätze: | 38-36/37-24/25 |
| WGK Germany | 2 |
| RTECS-Nr. | NY2975000 |
| F | 10-23 |
| HS Code | 29419090 |
| Giftige Stoffe Daten | 68-41-7(Hazardous Substances Data) |