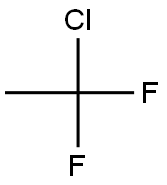
1-Chlor-1,1-difluorethan
Bezeichnung:1-Chlor-1,1-difluorethan
CAS-Nr75-68-3
Englisch Name:1-Chloro-1,1-difluoroethane
CBNumberCB8162234
SummenformelC2H3ClF2
Molgewicht100.5
MOL-Datei75-68-3.mol
Synonyma
1-Chlor-1,1-difluorethan
HCFC 142 b
1-Chlor-1,1-difluorethan physikalisch-chemischer Eigenschaften
| Schmelzpunkt | −131 °C(lit.) |
| Siedepunkt | −10 °C(lit.) |
| Dichte | 1.108 |
| Dampfdichte | 3.49 (vs air) |
| Dampfdruck | 2196 mm Hg ( 21 °C) |
| Brechungsindex | 1.3672 (estimate) |
| Explosionsgrenze | 18% |
| Stabilität | Stable. Highly flammable. Incompatible with strong oxidizing agents, metals - use brass regulators, steel cylinders for storage. |
| LogP | 1.330 (est) |
| CAS Datenbank | 75-68-3(CAS DataBase Reference) |
| NIST chemische Informationen | Ethane, 1-chloro-1,1-difluoro-(75-68-3) |
| EPA chemische Informationen | HCFC-142b (75-68-3) |
| Kennzeichnung gefährlicher | F+,N,Xi |
| R-Sätze: | 12-59 |
| S-Sätze: | 38-59 |
| RIDADR | UN 2517 2.1 |
| WGK Germany | 1 |
| RTECS-Nr. | KH7650000 |
| Hazard Note | Irritant |
| DOT Classification | 2.1 (Flammable gas) |
| HazardClass | 2.1 |
| HS Code | 29033990 |
| Giftige Stoffe Daten | 75-68-3(Hazardous Substances Data) |
| Toxizität | LC50 inhalation in mouse: 1758gm/m3/2H |


