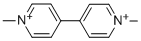
1,1'-Dimethyl-4,4'-bipyridinium-Salze
Bezeichnung:1,1'-Dimethyl-4,4'-bipyridinium-Salze
CAS-Nr4685-14-7
Englisch Name:Paraquat
CBNumberCB7512162
SummenformelC12H14N2+2
Molgewicht186.25
MOL-Datei4685-14-7.mol
Synonyma
1,1'-Dimethyl-4,4'-bipyridinium
1,1'-Dimethyl-4,4'-bipyridinium-Salze
1,1'-Dimethyl-4,4'-bipyridinium-Salze physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 300 °C (decomp) |
| Siedepunkt | 310.75°C (rough estimate) |
| Dichte | 0.9748 (rough estimate) |
| Brechungsindex | 1.5600 (estimate) |
| Aggregatzustand | Yellow solid |
| Farbe | Off-white Powder |
| EPA chemische Informationen | Paraquat (4685-14-7) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 26/27/28 |
| S-Sätze: | 1-13-45 |
| RIDADR | 2781 |
| OEB | D |
| OEL | TWA: 0.1 mg/m3 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| Giftige Stoffe Daten | 4685-14-7(Hazardous Substances Data) |
| Toxizität | LD50 orl-rat: 100 mg/kg IYKEDH 10,520,79 |
| Toxizität | LD50 orl-rat: 150 mg/kg FMCHA2 -,C118,83 |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)



-
Alarmwort
Achtung
-
Gefahrenhinweise
H290:Kann gegenüber Metallen korrosiv sein.
H301:Giftig bei Verschlucken.
H311:Giftig bei Hautkontakt.
H315:Verursacht Hautreizungen.
H319:Verursacht schwere Augenreizung.
H330:Lebensgefahr bei Einatmen.
H335:Kann die Atemwege reizen.
H372:Schädigt bei Hautkontakt und Verschlucken die Organe bei längerer oder wiederholter Exposition.
H410:Sehr giftig für Wasserorganismen mit langfristiger Wirkung.
-
Sicherheit
P234:Nur im Originalbehälter aufbewahren.
P260:Dampf/Aerosol/Nebel nicht einatmen.
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P270:Bei Gebrauch nicht essen, trinken oder rauchen.
P271:Nur im Freien oder in gut belüfteten Räumen verwenden.
P273:Freisetzung in die Umwelt vermeiden.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P284:Atemschutz tragen.
P301+P310:BEI VERSCHLUCKEN: Sofort GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P304+P340:BEI EINATMEN: Die Person an die frische Luft bringen und für ungehinderte Atmung sorgen.
P305+P351+P338:BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen.
P310:Sofort GIFTINFORMATIONSZENTRUM/Arzt/ anrufen.
P312:Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P314:Bei Unwohlsein ärztlichen Rat einholen / ärztliche Hilfe hinzuziehen.
P320:Besondere Behandlung dringend erforderlich
P321:Besondere Behandlung
P322:Gezielte Maßnahmen
P330:Mund ausspülen.
P332+P313:Bei Hautreizung: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
P361:Alle kontaminierten Kleidungsstücke sofort ausziehen.
P362:Kontaminierte Kleidung ausziehen und vor erneutem Tragen waschen.
P363:Kontaminierte Kleidung vor erneutem Tragen waschen.
P390:Verschüttete Mengen aufnehmen, um Materialschäden zu vermeiden.
P391:Verschüttete Mengen aufnehmen.
P403+P233:An einem gut belüfteten Ort aufbewahren. Behälter dicht verschlossen halten.
P404:In einem geschlossenen Behälter aufbewahren.
P405:Unter Verschluss aufbewahren.
P501:Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen.
Paraquat Chemische Eigenschaften,Einsatz,Produktion Methoden
-
R-Sätze Betriebsanweisung:
R26/27/28:Sehr giftig beim Einatmen, Verschlucken und Berührung mit der Haut. -
S-Sätze Betriebsanweisung:
S1:Unter Verschluss aufbewahren.
S13:Von Nahrungsmitteln, Getränken und Futtermitteln fernhalten.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen). -
Beschreibung
Paraquat is a quaternary ammonium compound with herbicide properties, as diquat. It is contained in Cekuquat or Dipril. -
Chemische Eigenschaften
Paraquat is a yellow solid with a faint, ammonia-like odor. -
Verwenden
It can cause contact and phototoxic contact dermatitis, acne, and leucoderma, mainly in agrieultural workers. -
Verwenden
Herbicide -
synthetische
Paraquat is synthesized by the direct quaternization of 4,4 -bipyridyl with chloromethane under pressure (1,9). Diquat is synthesized by reaction of 2,2 -bipridyl with di-n-propyl amine. -
Vorbereitung Methode
Paraquat is produced in several countries by coupling pyridine in the presence of sodium in anhydrous ammonia and quaternizing the resulting 4,4-bipyridyl with methyl chloride. The only impurity permitted is the 4,40-bipyridyl at a maximum level of 0.25% of the paraquat content. It is formulated for commercial use in various concentrations and mixtures. In the United States, paraquat is sold in watersoluble concentrates at 1.5–2.5 lb/gal. -
Definition
ChEBI: An organic cation that consists of 4,4'-bipyridine bearing two N-methyl substituents loctated at the 1- and 1'-positions. -
Health Hazard
Paraquat salts show moderate to high acutetoxicity among species, the oral LD50 values ranging between 25 and 300 mg/kg.The LD50 values in dogs, cats, guinea pigs,and rats are 25, 35, 30, and 100 mg/kg,respectively, for the sulfate salts. The toxicsymptoms from ingestion and inhalationof dusts include headache, nausea, vomiting, diarrhea, ulceration, dyspnea, and lunginjury. Single exposure to paraquat aerosols 3–5 mm in diameter at 1 mg/m3 concentration for 6 hours caused death to rats (ACGIH 1986). Inhalation of nonrespirable size dustscaused intense irritation and nose bleeding.Repeated exposures can lead to severe pulmonary edema and fibrosis. The dusts are anirritant to the respiratory tract.Dey and associates (1990) investigatedparaquat pharmacokinetics in rats, using asubcutaneous toxic low dose (72 mmol/kg)of [14CH3] paraquat, which would producelung disease but no renal damage. Paraquatwas rapidly absorbed. Peak blood concentrations were 58 nmol/mL at 20 minutes,while its peak concentrations in the lungand kidney were 65 and 359 nmol/g, respectively, at 40 minutes. About 85% of dosewas eliminated in the urine by 7 days. Of theremaining radioactivity, 79% remained in thebody and 21% remained in the lung, causing progressive lung disease. Excretion ofthe retained amount was slow and prolonged.Chui and coworkers (1988) investigated thetoxicokinetics of paraquat and the effects ofdifferent routes of administration. A singledose of 11.4 mg/kg of [14CH3 ]paraquat wasadministered in rats by intravenous, intragastric, dermal, and pulmonary (exposure byaqueous or liquid aerosols) routes. The majorexcretion routes were urine and feces.The radioactivity absorbed into thesystemic circulation of the rat was about27.5, 23.8, 8.5, and 1.5 nmol for inhalationthrough a tracheal cannula, nose-only expo-sure, intragastric injection, and dermalabsorption, respectively. The bulk of theherbicide administered by the inhalation anddermal routes remained at the sites of theadministration.In humans, cardiovascular collapse result-ing from acute paraquat poisoning is asso-ciated with the distribution phase; the lateoccurrence of death-related pulmonary fibro-sis is associated with the elimination phase(Houze et al. 1990). Toxicokinetics studiesconducted by these investigators in acutehuman poisoning cases indicated a concen-tration of paraquat in blood that had a mean distribution half-life of 5 hours and a meaneliminationhalf-lifeof84 hours,respectively.It was excreted in the urine. It was retainedin the muscle for several weeks after poi-soning. Electron microscopy studies (TEMand SEM) on the lungs obtained from adog after 7 days of intravenous administra-tion of paraquat (12 mg/kg) indicate detach-ment of alveolar epithelial cells and alve-olar macrophage, which plays a significantrole in paraquat-induced pulmonary fibrosis(Hampson and Pond 1988). Administrationof paraquat to adult rat pulmonary alveolarmacrophages in primary culture caused celldeaththatwasdependentonthedoseandtime.The cell death was potentiated by hyperoxia(95% O) and extracellular production of anactive oxygen species, the superoxide anionradical (Wong and Stevens 1985). Althoughdiquat can enter the cells to a greater extent,than can paraquat, the latter is about twice aspotent as diquat (Wong and Stevens 1986).Paraquat is a urinary metabolite of 4,48-bipyridyl when the latter is administeredintraperitoneally to guinea pigs. Godin andCrooks (1989) detected 2.9% N-methyl-4,48-bipyridinium ion in the urine of animalstreated with 4,48-bipyridyl, thus indicat-ing the formation of such toxic metabolitesthrough the N-methylation pathway.Certain substances have been reported topotentiate the toxicity of paraquat. Theseinclude transition metal ions such as copper(Kohen and Chevion 1985) and ethanol (Kuoand Nanikawa 1990). Blood paraquat lev-els showed significant elevation in rabbits,and the mortality rates increased when t -
Landwirtschaftliche Anwendung
Herbicide, Defoliant, Desiccant: Paraquat is a quaternary nitrogen herbicide widely used for broadleaf weed control. It is a quick-acting, nonselective compound, that destroys green plant tissue on contact and by translocation within the plant. It has been employed for killing marijuana in the U.S. and in Mexico. It is also used as a crop desiccant and defoliant, and as an aquatic herbicide. It is used in many formulations with other herbicides, e. g., simazine and diquat. Paraquat dichloride is registered for the control of weeds and grasses in agricultural and non-agricultural areas. It is used as a preplant or pre-emergence herbicide on vegetables, grains, cotton, grasses, sugarcane, peanuts, potatoes, and on areas for tree plantation establishment. Paraquat is applied as a directed spray post-emergence herbicide around fruit crops, vegetables, trees, vines, grains, soybeans, and sugarcane. It is used for dormant season applications on clover and other legumes, and for chemical fallow. It is also used as a desiccant or harvest aid on cotton, dry beans, soybeans, potatoes, sunflowers, sugarcane and as a post-harvest desiccant on tomatoes. It is applied to pine trees to induce resin soaking. Paraquat dichloride is also used on non-crop areas such as public airports, electric transformer stations and around commercial buildings to control weeds. Not approved for use in EU countries. A U.S. EPA restricted Use Pesticide (RUP). -
Handelsname
HERBICIDE®[C]; CRISQUAT®; CYCLONE®; DEXTRONE®; DEXTRONE-X®; ESGRAM®; GAMIXEL®; GOLDQUAT 276®; GRAMOXONE®; GRAMOXONE D®; GRAMOXONE DICHLORIDE®; GRAMOXONE S®; GRAMOXONE W®; HERBIKILL®; HERBOXONE®; OK 622®; ORTHO PARAQUAT CL®[C]; PARA-COL®; PARAMINE®; PARAQUAT CL®[C]; PARAQUAT DICHLORIDE BIPYRIDYLNIUM HERBICIDE®; PATHCLEAR®; PILLARQUAT®; PILLARXONE®; PP148®; PRELUDE®[C]; STARFIRE®; SUREFIRE®; SWEEP®; TERRAKLENE®; TOTACOL®; TOXER TOTAL®; UNIQUAT®; WEEDOL® -
Kontakt-Allergie
Paraquat is a quaternary ammonium compound with herbicide properties, as diquat. It is contained in Cekuquat? or Dipril?. It can cause contact and phototoxic contact dermatitis, acne, and leukoderma mainly in agricultural workers. -
Sicherheitsprofil
Poison by ingestion and intraperitoneal routes. Mutation data reported. Human systemic effects: changes in structure or function of esophagus, darrhea, edema, fibrosis of lung, fibrosis, focal (pneumoconiosis), hemorrhage, jaundice, renal damage, renal function tests depressed, respiratory depression, ulceration or bleedmg from stomach, vomiting. Death from anoxia may result. When heated to decomposition it emits toxic fumes of NOx. See also PARAQUAT DICHLOFUDE -
mögliche Exposition
Those engaged in manufacture, formulation and application of this herbicide. Classified for restricted use: limited to use by a certified applicator, or those under applicator’s direct supervision. -
Carcinogenicity
Several carcinogenicity studies have been conducted on paraquat by the oral route. In a 2-year feeding study at doses as high as 75 mg/kg in mice, and a drinking water study at doses as high as 2.6 mg/L of water in rats, no evidence of tumorigenicity was seen. Similar negative results were reported for diquat in a 2-year feeding study (Hayes, 1991) in rats at dose levels up to 720 mg/kg and in a 2-year drinking water study in mice at doses of 2–4 mg/kg. -
Stoffwechsel
Because paraquat and diquat are positively charged ions, they are very quickly and tightly bound to negatively charged clay particles in the soil, rendering them totally inactive (1). Thus, these herbicides have no soil activity.
Plants do not actively metabolize either paraquat or diquat (31); however, substantial photodegradation does occur on the leaf surface. Isonicotinic acid and methylamine hydrochloride are the decomposition products most often noted from paraquat (1). With diquat, the decomposition products included the pyrazinium salt, picolinamide, and picolinic acid (1). Photodegradation of diquat was greater than that of paraquat at equal irradiances (1–3). -
Versand/Shipping
UN2781 Bipyridilium pesticide, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. -
Toxicity evaluation
Paraquat is most active in rapidly respiring tissue and in the presence of oxygen, it is not surprising that most of the damage in mammalian systems is associated with lung tissue. Symptoms such as fibrosis and hemorrhage of the lungs are often detected after paraquat poisoning. -
Inkompatibilitäten
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides (hydrolysis), alkylarylsulfonate wetting agents. Corrosive to metals. Decomposes in presence of ultraviolet (UV) light. Decomposes in heat (see physical properties, above) and in the presence of UV light, producing nitrogen oxides, hydrogen chloride. -
Waste disposal
Paraquat is rapidly inactivated in soil. It is also inactivated by anionic surfactants. Therefore an effective and environmentally safe disposal method would be to mix the product with ordinary household detergent and bury the mixture in clay soil. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
1,1'-Dimethyl-4,4'-bipyridinium-Salze Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(87)Suppliers
-
Henan Tianfu Chemical Co.,Ltd.
Telefon +86-0371-55170693<br/>+86-19937530512
E-Mail info@tianfuchem.com
-
Telefon +86-0371-86658258<br/>+8613203830695
E-Mail sales@coreychem.com
-
Hubei Jusheng Technology Co.,Ltd.
Telefon 18871490254
E-Mail linda@hubeijusheng.com
-
Telefon +86-86-5926051114<br/>+8615060885618
E-Mail sales@amoychem.com
-
Hubei xin bonus chemical co. LTD
Telefon 86-13657291602
E-Mail linda@hubeijusheng.com
-
Telefon +86-023-6139-8061<br/>+86-86-13650506873
E-Mail sales@chemdad.com
-
Hefei TNJ Chemical Industry Co.,Ltd.
Telefon +86-0551-65418671<br/>+8618949823763
E-Mail sales@tnjchem.com
-
Wuhan Monad Medicine Tech Co.,LTD
Telefon 02768782018<br/>18771942761
E-Mail sales01@whmonad.com
-
SUZHOU SENFEIDA CHEMICAL CO.,LTD
Telefon +86-0512-83500002<br/>+8615195660023
E-Mail sales@senfeida.com
-
Shanghai Likang New Materials Co., Limited
Telefon +86-16631818819<br/>+86-17736933208
E-Mail 3684455296@qq.com
1of2
