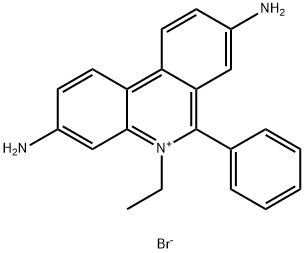
3,8-Diamino-1-ethyl-6-phenylphe-nantridinium-bromid
Bezeichnung:3,8-Diamino-1-ethyl-6-phenylphe-nantridinium-bromid
CAS-Nr1239-45-8
Englisch Name:Ethidium bromide
CBNumberCB7406991
SummenformelC21H20BrN3
Molgewicht394.32
MOL-Datei1239-45-8.mol
Synonyma
3,8-Diamino-1-ethyl-6-phenylphenantridiniumbromid
3,8-Diamino-1-ethyl-6-phenylphe-nantridinium-bromid
3,8-Diamino-5-ethyl-6-phenylphenanthridiniumbromid
3,8-Diamino-1-ethyl-6-phenylphe-nantridinium-bromid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 260-262 °C (dec.)(lit.) |
| Dichte | 1.3739 (rough estimate) |
| chüttdichte | 340kg/m3 |
| Brechungsindex | 1.6700 (estimate) |
| Flammpunkt | >100°C |
| storage temp. | 2-8°C |
| Löslichkeit | H2O: 10 mg/mL, opaque, strongly red |
| Aggregatzustand | powder |
| Farbe | Red to dark purple |
| Geruch (Odor) | Odorless solid |
| PH | 4.4 (20°C, 10g/L in H2O) |
| Wasserlöslichkeit | 40 g/L (25 ºC) |
| maximale Wellenlänge (λmax) | 518 nm, 210 nm, 285 nm, 316 nm, 343 nm, 480 nm, 525 nm |
| Merck | 14,4731 |
| BRN | 3642536 |
| Stabilität | Stable. Incompatible with strong oxidizing agents. |
| Biologische Anwendungen | Nucleic acid hybridization; detecting nucleic acids,cells,cancer cells,human cytomegalovirus,hydrogenase A (hydA) of Clostridia,influenza A virus,oligonucleotides,viable Plesiomonas shigelloides; apoptosis assay; nucleic acid quantification |
| Major Application | Electroluminescent displays;photoresists |
| Kennzeichnung gefährlicher | T,T+ |
| R-Sätze: | 23-68-36/37/38-26-21/22-22 |
| S-Sätze: | 36/37-45-36/37/39-28A-26-22-28-63 |
| RIDADR | UN 2811 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS-Nr. | SF7950000 |
| F | 8-9 |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 29339990 |
| Giftige Stoffe Daten | 1239-45-8(Hazardous Substances Data) |
| Toxizität | Intercalating dye widely used to stain DNA in gels and gradients. The DNA can be visualized readily by irradiation with ultraviolet light, as little as 0.5 μg of DNA being detectable by such methods. Ethidium bromide is also added to cesium chloride density gradients, since as an intercalating molecule it binds more readily to linear DNA than to closed collinear circles of DNA (such as plasmids). Binding of ethidium bromide reduces the density of DNA, thus covalent circles of DNA have higher densities at saturating concentrations of ethidium bromide, permitting the separation of plasmid DNA. Ethidium bromide is a putative carcinogen. |


