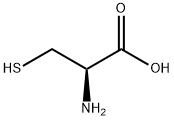
L-Cystein
Bezeichnung:L-Cystein
CAS-Nr52-90-4
Englisch Name:L-Cysteine
CBNumberCB7388480
SummenformelC3H7NO2S
Molgewicht121.16
MOL-Datei52-90-4.mol
Synonyma
L-Cystein
L-Cystein physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 240 °C (dec.) (lit.) |
| alpha | 8.75 º (c=12, 2N HCl) |
| Siedepunkt | 293.9±35.0 °C(Predicted) |
| Dichte | 1.197 (estimate) |
| chüttdichte | 300kg/m3 |
| FEMA | 3263 | L-CYSTEINE |
| Brechungsindex | 8.8 ° (C=8, 1mol/L HCl) |
| storage temp. | Store below +30°C. |
| Löslichkeit | H2O: 25 mg/mL |
| Aggregatzustand | Solid |
| pka | 1.92(at 25℃) |
| Farbe | White |
| PH | 4.5-5.5 (100g/l, H2O, 20℃) |
| Geruch (Odor) | sulfury |
| Geruchsart | sulfurous |
| Biologische Quelle | non-animal source |
| Optische Aktivität | Optical rotation: +8° to +9° (c = 5, 1 N HCl, 20°C). |
| Wasserlöslichkeit | 280 g/L (25 ºC) |
| Kennzeichnung gefährlicher | Xn,Xi |
| R-Sätze: | 22-36/37/38-20/21/22 |
| S-Sätze: | 36-37/39-26-24/25 |
| WGK Germany | 3 |
| RTECS-Nr. | HA1600000 |
| F | 10-23 |
| Selbstentzündungstemperatur | 420 °C |
| TSCA | Yes |
| HS Code | 29309012 |
| Giftige Stoffe Daten | 52-90-4(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 1890 mg/kg |

