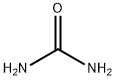
Harnstoff
Bezeichnung:Harnstoff
CAS-Nr57-13-6
Englisch Name:Urea
CBNumberCB5853861
SummenformelCH4N2O
Molgewicht60.06
MOL-Datei57-13-6.mol
Synonyma
Harnstoff
Carbamid
Carbonyldiamid
Urea
Harnstoff physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 132-135 °C(lit.) |
| Siedepunkt | 332.48°C (estimate) |
| Dichte | 1.335 g/mL at 25 °C(lit.) |
| chüttdichte | 720-760kg/m3 |
| Dampfdruck | <0.1 hPa (20 °C) |
| Brechungsindex | n |
| storage temp. | 2-8°C |
| Löslichkeit | H2O: 8 M at 20 °C |
| Aggregatzustand | powder |
| pka | 0.10(at 25℃) |
| Farbe | white |
| Wichte | 1.335 |
| Geruch (Odor) | almost odorless |
| PH | 8.0-10.0 (20℃, 8M in H2O) |
| Biologische Quelle | synthetic |
| Wasserlöslichkeit | 1080 g/L (20 ºC) |
| maximale Wellenlänge (λmax) | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,9867 |
| BRN | 635724 |
| Dielectric constant | 3.5(Ambient) |
| Stabilität | Substances to be avoided include strong oxidizing agents. Protect from moisture. |
| InChIKey | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
| LogP | -1.660 (est) |
| CAS Datenbank | 57-13-6(CAS DataBase Reference) |
| NIST chemische Informationen | Urea(57-13-6) |
| EPA chemische Informationen | Urea (57-13-6) |
| Absorption | ≤0.06 at 260nm at 5M ≤0.06 at 280nm at 5M |
| Kennzeichnung gefährlicher | Xn,Xi |
| R-Sätze: | 36/37/38-40-38 |
| S-Sätze: | 26-36-24/25-37 |
| RIDADR | Not regulated |
| WGK Germany | 1 |
| RTECS-Nr. | YR6250000 |
| TSCA | Yes |
| HS Code | 31021010 |
| Giftige Stoffe Daten | 57-13-6(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 8471 mg/kg LD50 dermal Rat 8200 mg/kg |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)

-
Alarmwort
Warnung
-
Gefahrenhinweise
H320:Causes eye irritation
-
Sicherheit
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P305+P351+P338:BEI KONTAKT MIT DEN AUGEN: Einige Minuten lang behutsam mit Wasser spülen. Eventuell vorhandene Kontaktlinsen nach Möglichkeit entfernen. Weiter spülen.
P337+P313:Bei anhaltender Augenreizung: Ärztlichen Rat einholen/ärztliche Hilfe hinzuziehen.
Urea Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
WEISSE KRISTALLE MIT CHARAKTERISTISCHEM GERUCH. -
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen über den Schmelzpunkt unter Bildung giftiger Gase. Reagiert sehr heftig mit starken Oxidationsmitteln, Nitriten, anorganischen Chloriden, Chloriten und Perchloraten unter Feuer- und Explosionsgefahr. -
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt (ACGIH 2005).
MAK nicht festgelegt (DFG 2005).
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation des Aerosols und durch Verschlucken. -
INHALATIONSGEFAHREN
Verdampfen bei 20°C vernachlässigbar; eine belästigende Partikelkonzentration in der Luft kann jedoch schnell erreicht werden, vor allem als Pulver. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen, die Haut und die Atemwege. -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Hautkontakt kann Dermatitis hervorrufen. -
LECKAGE
Verschüttetes Material in Behältern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste mit viel Wasser wegspülen. -
R-Sätze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R40:Verdacht auf krebserzeugende Wirkung. -
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S24/25:Berührung mit den Augen und der Haut vermeiden. -
Beschreibung
Urea is a stable highly water-soluble compound of high nitrogen content (47%), with good storage properties that make it the most commonly used nitrogen fertilizer. The synthesis process has remained essentially unchanged since it was first developed by the BASF Corporation in 1922. In this process, liquid ammonia is reacted with carbon dioxide to produce ammonium carbamate, which is then dehydrated to form urea. The reactions are:
2NH3 + CO2 ===? NH2·CO2·NH4
NH2·CO2·NH4 ===? (NH2)2CO + H2O
-
Chemische Eigenschaften
Urea is a white crystalline so lid. -
Chemische Eigenschaften
Urea,CO(HN2)2, also known as carbamide, is a white crystalline powder that has a melting point of l32.7 °C (270 °F). It is a natural product of animal protein metabolism and is the chief nitrogen constituent of urine. Commercially, urea is produced by the reaction of ammonia and carbon dioxide. It is soluble in water, alcohol, and benzene. -
Occurrence
The compound was discovered by Hilaire Rouelle in 1773 as a constituent of urine. -
History
Urea has the distinction of being the first synthesized organic compound. Until the mid-18th century, scientists believed organic compounds came only from live plants and animals. The first serious blow to the theory of vitalism, which marked the beginning of modern organic chemistry, occurred when Friedrich W?hler (1800 1882) synthesized urea from the two inorganic substances, lead cyanate and ammonium hydroxide: Pb(OCN)2 + 2NH4OH→2(NH2)2CO + Pb(OH)2. W?hler's discoveries on urea occurred while he was studying cyanates; he was attempting to synthesize ammonium cyanate when he discovered crystals of urea in his samples. He first prepared urea in 1824, but he did not identify this product and report his findings until 1828. W?hler's synthesis of urea signaled the birth of organic chemistry. -
Verwenden
The primary use of urea is as a nitrogen source in fertilizers, with about 90% of the urea production being used for this purpose. Urea's high nitrogen content (46%) makes it a concentrated source for adding fixed nitrogen to soils. It can be applied to the soil alone, but its high nitrogen content can stress plants and impact the soil negatively, so it is often blended with other nutrients. Blending also reduces the nitrogen content of the fertilizer. For example, blending with ammonium nitrate, NH4NO3, in different proportions produces fertilizers with various nitrogen contents. Urea in the soil is converted to ammonium nitrogen and taken up by plants. It can be applied in solid granule form or dissolved in water and used as a spray. Urea is also used agriculturally as a supplement in livestock feeds to assist protein synthesis.
Another use of urea is for resins, which are used in numerous applications including plastics, adhesives, moldings, laminates, plywood, particleboard, textiles, and coatings. Resins are organic liquid substances exuded from plants that harden on exposure to air. The term now includes numerous synthetically produced resins. Urea resins are thermosetting, which means they harden when heated, often with the aid of a catalyst. The polymerization of urea and formaldehyde produces urea-formaldehyde resins, which is the second most abundant use of urea. Urea is dehydrated to melamine, which, when combined with formaldehyde, produces melamine-formaldehyde resins. -
Verwenden
Urea is a physiological regulator of nitrogen excretion in mammals; synthesized in the liver as an end-product of protein catabolism and excreted in urine. Also occurs normally in skin. Emollient; diu retic. -
Verwenden
1) UREA, FCC is an odorless and colorless solid that is an important nitrogen-containing substance found in mammal urine.2) Urea has little or no nutritional value to monogastric mammals but Urea is used in sugar-free chewing gum to adjust the texture -
Verwenden
anticholelithogenic; LD50(rat) 890 mg/kg ip -
Verwenden
Used for the denaturation of proteins and as a mild solubilization agent for insoluble or denatured proteins. Useful for renaturing proteins from samples already denatured with 6 M guanidine chloride such as inclusion bodies. May be used with guanidine hydrochloride and dithiothreitrol (DTT) in the refolding of denatured proteins into their native or active form. -
Verwenden
urea is incorporated into cosmetics for a variety of purposes, including moisturizing, desquamating, anti-microbial, and buffering. urea is regarded as a “true” moisturizer rather than a humectant because it attracts and retains moisture in the corneum layer. It facilitates the natural exfoliation of keratinocytes given its ability to dissolve intercellular cement in the corneum layer. Through its anti-microbial properties that inhibit the growth of micro-organisms in a product, urea can also be part of a larger preservative system. This ingredient’s buffering action is attributed to its ability to regulate the hydrolipid mantle. In addition, urea is found to enhance the penetration and absorption of other active ingredients, relieve itchiness, and help leave the skin feeling soft and supple. Anti-inflammatory, anti-septic, and deodorizing actions allow it to protect the skin’s surface against negative changes and help maintain healthy skin. Studies show that urea does not induce photoallergy, phototoxicity, or sensitization. The safest concentration of use in skin care preparations is between 2 and 8 percent. High concentrations of urea seem to be unstable when incorporated into skin care preparations and can also cause irritation. Acidic urea solutions can produce burning or stinging sensations. -
Vorbereitung Methode
Urea is an important industrial compound. The synthesis of urea was discovered in 1870.Commercial production of urea involves the reaction of carbon dioxide and ammonia at highpressure and temperature to produce ammonium carbamate. Ammonium carbamate is thendehydrated to produce urea (Figure 96.1). The reaction uses a molar ratio of ammonia tocarbon dioxide that is approximately 3:1 and is carried out at pressures of approximately 150atmospheres and temperatures of approximately 180°C. -
Definition
A white crystalline compound made from ammonia and carbon dioxide. It is used in the manufacture of urea–formaldehyde (methanal) resins. Urea is the end product of metabolism in many animals and is present in urine. -
Definition
ChEBI: A carbonyl group with two C-bound amine groups. -
Indications
Urea-containing preparations have a softening and moisturizing effect on the stratum corneum and, at times, may provide good therapy for dry skin and the pruritus associated with it. They appear to have an antipruritic effect apart from their hydrating qualities. Urea compounds disrupt the normal hydrogen bonds of epidermal proteins; therefore, their effect in dry hyperkeratotic diseases such as ichthyosis vulgaris and psoriasis is not only to make the skin more pliable but also to help remove adherent scales. Lactic acid also has a softening and moisturizing effect on the stratum corneum.
Urea 40% ointment may be useful in removing hypertrophic or dystrophic psoriatic nails. Subsequent topical therapy to the denuded nail bed and proximal nail fold may result in regrowth of ‘‘normal’’ nails in half of those treated. -
synthetische
All current processes for the manufacture of urea are based on the reaction of ammonia and carbon dioxide to form ammonium carbamate which is simultaneously dehydrated to urea:
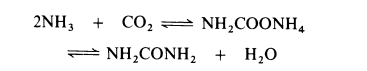
The dehydration of ammonium carbamate is appreciable only at temperatures above the melting point (about 150??C) and this reaction can only proceed if the combined partial pressure of ammonia and carbon dioxide exceeds the dissociation pressure of the ammonium carbamate (about 10 MPa at 160??C and about 30 MPa at 200??C). Thus commercial processes are operated in the liquid phase at 160-220??C and 18-35 MPa (180-350 atmospheres). Generally, a stoichiometric excess of ammonia is employed, molar ratios of up to 6: 1 being used. The dehydration of ammonium carbamate to urea proceeds to about 50-65% in most processes. The reactor effluent therefore consists of urea, water, ammonium carbamate and the excess of ammonia. Various techniques are used for separating the components. In one process the effluent is let down in pressure and heated at about 155??C to decompose the carbamate into ammonia and carbon dioxide. The gases are removed and cooled. All the carbon dioxide present reacts with the stoichiometric amount of ammonia to re-form carbamate, which is then dissolved in a small quantity of water and returned to the reactor. The remaining ammonia is liquefied and recycled to the reactor. Fresh make-up ammonia and carbon dioxide are also introduced into the reactor. Removal of ammonium carbamate and ammonia from the reactor effluent leaves an aqueous solution of urea. The solution is partially evaporated and then urea is isolated by recrystallization. Ammonium carbamate is very corrosive and at one time it was necessary to use silver-lined equipment but now satisfactory alloy steel plant is available. -
Trademarks
Ureaphil (Hospira). -
Biologische Funktion
The use of urea (Ureaphil, Urevert) has declined in recent years owing both to its disagreeable taste and to the increasing use of mannitol for the same purposes. When used to reduce cerebrospinal fluid pressure, urea is generally given by intravenous drip. Because of its potential to expand the extracellular fluid volume, urea is contraindicated in patients with severe impairment of renal, hepatic, or cardiac function or active intracranial bleeding. -
Allgemeine Beschreibung
Solid odorless white crystals or pellets. Density 1.335 g /cc. Noncombustible. -
Air & Water Reaktionen
Water soluble. -
Reaktivität anzeigen
Urea is a weak base. Reacts with hypochlorites to form nitrogen trichloride which explodes spontaneously in air [J. Am. Chem. Soc. 63:3530-32]. Same is true for phosphorus pentachloride. Urea reacts with azo and diazo compounds to generate toxic gases. Reacts with strong reducing agents to form flammable gases (hydrogen). The heating of improper stoichiometric amounts of Urea and sodium nitrite lead to an explosion. Heated mixtures of oxalic acid and Urea yielded rapid evolution of gases, carbon dioxide, carbon monoxide and ammonia (if hot, can be explosive). Titanium tetrachloride and Urea slowly formed a complex during 6 weeks at 80°C., decomposed violently at 90°C., [Chem. Abs., 1966, 64, 9219b]. Urea ignites spontaneously on stirring with nitrosyl perchlorate, (due to the formation of the diazonium perchlorate). Oxalic acid and Urea react at high temperatures to form toxic and flammable ammonia and carbon monoxide gasses, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42]. -
Health Hazard
May irritate eyes. -
Brandgefahr
Behavior in Fire: Melts and decomposes, generating ammonia. -
Landwirtschaftliche Anwendung
Urea, CO(NH2)2, also referred to as carbamide, is a white, crystalline, organic, water-soluble fertilizer. It contains around 46 % nitrogen, the highest N percentage any solid fertilizer can have.
Apart from its major use as a fertilizer, urea is also employed in the manufacture of paints, glues, plastics, paper, textiles, feed and weed control chemicals as well as a source of non-protein nitrogen.
Urea is an acceptable fertilizer for rice and preferable to nitrates for flooded rice because of the reduction of nitrates to N,O and/or nitrogen (in anaerobic conditions) which is lost to the atmosphere. Also, rice can utilize the ammonium form of nitrogen efficiently. Hydrolysis and nitrification (in aerobic conditions) are rapid in tropical, sub-tropical and warm climates.
Urea can thus be used efficiently but its use requires a better understanding than that required for other inorganic salts. It is applied to flooded soil three times: at the time of planting, tillering and panicle development. Similar to other nitrogenous fertilizers, urea promotes the growth of both weeds and crops. Urea solution after evaporation in vacuum evaporators, can be finally spraydried into pellets or prills. When protected from moisture (to which it is susceptible), urea is non-caking, freeflowing and suitable for storage and handling.
However, the benefits of urea outweigh its disadvantages. Insofar as the weed growth is concerned, effective methods should be devised to minimize it to a manageable level.
Urea is converted rapidly to ammonia by hydrolysis in the soil via the ammonium carbonate formation route, the latter being unstable (decomposing to ammonia and carbon dioxide). Urea is not as quick acting as ammonium nitrate because the nitrifying bacteria require a few days of warm and moist soil conditions to convert ammonia to the nitrate form. The formation of ammonium ion is slightly acidic in its ultimate reaction with the soil.
Urea is decomposed by the enzyme urease and a part of urea is lost as gaseous nitrogen. The time between urea application and the first availability of water to the soil is important, as also the temperature, because the enzyme is less reactive in cold than at high temperature (25 to 30°C). Prevention and retardation of the hydrolytic action of urease is important following the addition of urea to soil. This may help to avoid difficulties associated with ammonia formation and alkalization.
Many substances are urease inhibitors, but very few meet the rather specific requirements of being (a) effective at low concentrations, (b) relatively non-toxic to higher forms of life, (c) inexpensive, and (d) compatible with urea.
Urea can be sprayed on leaves and can also be mixed with insecticides or herbicides for soil application. A urea-ammonium nitrate mixture with herbicide is also used for weed control.
Urea, although an excellent fertilizer, suffers from the following drawbacks: (i) When applied to a bare soil surface, urea hydrolyzes rapidly and loses a significant quantity of ammonia by volatilization. Such losses vary from soil to soil and are greater for urea in a pellet form rather than in a solution form. Burning residues on the field is suggested as a practical means to control the ammonia loss because the burning reduces the concentration of the enzyme urease in plants. (ii) Rapid hydrolysis of urea in soils can cause injury to the seedlings by ammonia, if large quantities of the fertilizer are placed too close to the seeds. (iii) The fertilizer grade urea may contain toxic biuret which is formed during urea manufacture by an excessive temperature rise. A large concentration of biuret in urea ( > 2 %) causes injury to plants. Feed-grade urea is sometimes referred to by the number 262 which is the product of its nitrogen content (42%) multiplied by 6.25, the latter being the factor used by chemists to convert nitrogen to its protein equiva -
Landwirtschaftliche Anwendung
Fertilizer, Fungicide: Used in fertilizers and animal feeds, as a fungicide, in the manufacture of resins and plastics, as a stabilizer in explosives and in medicines, and others. Urea is used to protect against frost and is used in some pesticides as an inert ingredient as a stabilizer, as an inhibitor and as an intensifier for herbicides. Registered for use in EU countries . Registered for use in the U.S. -
Handelsname
PRESPERSION, 75 UREA®; SUPERCEL 3000®; UREAPHIL®; UREOPHIL®; UREVERT®; VARIOFORM II® -
Biochem/physiol Actions
Urea solution is primarily used for protein denaturation. It also increases solubility of hydrocarbons and reduce micelle formation. Urea solution at high concentration leads to the destabilization of amyloid β16?22 oligomers. -
Sicherheitsprofil
Moderately toxic by intravenous and subcutaneous routes. Human reproductive effects by intraplacental route: ferthty effects. Experimental reproductive effects. Human mutation data reported. A human skin irritant. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Reacts with sodium hypochlorite or calcium hypochlorite to form the explosive nitrogen trichloride. Incompatible with NaNO2, P2Cl5, nitrosyl perchlorate. Preparation of the 15N-labeled urea is hazardous. When heated to decomposition it emits toxic fumes of NOx. -
mögliche Exposition
Urea is used in ceramics, cosmetics, paper processing; resins, adhesives, in animal feeds; in the manufacture of isocyanurates; resins, and plastics; as a stabilizer in explosives; in medicines; anticholelithogenic, and others. -
Erste Hilfe
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. -
Environmental Fate
Terrestrial Fate
Urea is expected to have very high mobility in soil. Urea is not expected to volatilize from dry soil surfaces based on its vapor pressure. Various field and laboratory studies have demonstrated that urea degrades rapidly in most soils. Urea is rapidly hydrolyzed to ammonium ions through soil urease activity, which produces volatile gases, that is, ammonia and carbon dioxide. However, the rate of hydrolysis can be much slower, depending on the soil type, moisture content, and urea formulation.
Aquatic Fate
Urea is not expected to adsorb to suspended solids and sediments. Volatilization from water surfaces is not expected. Urea is rapidly hydrolyzed to ammonia and carbon dioxide in environmental systems by the extracellular enzyme urease, which originates from microorganisms and plant roots.
Atmospheric Fate
According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, urea, which has a vapor pressure of 1.2×10-5mm Hg at 251°C, will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase urea is degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 9.6 days. -
Stoffwechsel
The high analysis and good handling properties of urea have made it the leading nitrogen fertilizer, both as a source of nitrogen alone or when compounded with other materials in mixed fertilizers. Although an excellent source of nitrogen, urea can present problems unless properly managed; due to its rapid hydrolysis to ammonia, significant volatilization loss of this may occur if prilled or granular urea is applied to and left on the soil surface without timely incorporation. Mixtures of urea and ammonium nitrate for use in mixed fertilizers are also more highly hygroscopic than ammonium nitrate itself. -
Lager
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from oxidizers. Where possible, automatically transfer material fromstorage containers to process containers. -
läuterung methode
Crystallise urea twice from conductivity water using centrifugal drainage and keeping the temperature below 60o. The crystals are dried under vacuum at 55o for 6hours. Levy and Margouls [J Am Chem Soc 84 1345 1962] prepared a 9M solution in conductivity water (keeping the temperature below 25o) and, after filtering through a medium-porosity glass sinter, added an equal volume of absolute EtOH. The mixture was set aside at -27o for 2-3 days and filtered cold. The precipitate was washed with a small amount of EtOH and dried in air. Crystallisation from 70% EtOH between 40o and -9o has also been used. Ionic impurities such as ammonium isocyanate have been removed by treating the concentrated aqueous solution at 50o with Amberlite MB-1 cation-and anion-exchange resin, and allowing it to crystallise on evaporation. [Benesch et al. J Biol Chem 216 663 1955.] It can also be crystallised from MeOH or EtOH, and is dried under vacuum at room temperature. [Beilstein 3 H 42, 3 I 19, 3 II 35, 3 III 80.] -
Toxicity evaluation
The primary mechanism of toxicity appears to be inhibition of the citric acid cycle. It leads to blockade of electron transport and a decrease in energy production and cellular respiration, which leads to convulsions. -
Inkompatibilitäten
Violent reaction with strong oxidizers, chlorine, permanganates, dichromates, nitrites, inorganic chlorides; chlorites, and perchlorates. Contact with hypochlorites can result in the formation of explosive compounds. -
Waste disposal
Controlled incineration in equipment containing a scrubber or thermal unit to reduce nitrogen oxide emissions.
Urea Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1of3
Downstream Produkte
- Reactive Black KN-BN
- ureaformaldelyde resin UF
- Ethyl-1,2,3,4-tetrahydro-2,4-dioxopyrimidin-5-carboxylat
- 2,4-DICHLOROTHIENO[3,2-D]PYRIMIDINE
- 5,6-Dihydro-5-methyluracil
- 5-Nitro-2-furfurolsemicarbazon
- Tanning agent for white leather
- synthetic carbamider ring tanning agent No.1
- 1-(2-Aminoethyl)imidazolidin-2-on
- DIRECT FAST BLACK G
- 2-((Aminocarbonyl)oxy)-N,N,N-tri-methylethan-aminiumchlorid
- Dihydrouracil
- 2-[(4-Methoxy-2-nitrophenyl)azo]-N-(2-methoxyphenyl)-3-oxobutyramid
- 6-Aminouracil
- flame retardane ZR-01
- 2,4-Dichlorothieno[3,2-d]pyrimidine
- 2,6-Dichlor-4,8-dipiperidinopyrimido[5,4-d]pyrimidin
- biodegrddable finishing agent for fabric
- 1,1-Hydrazoformamid
- 1-(1,3-DIHYDRO-1-OXOISOBENZOFURAN-3-YL)UREA
- N,N''-(Isobutyliden)diharnstoff
- N-Methyl-N-nitrosoharnstoff
- 4,5-Dihydroxy-1,3-bis(hydroxymethyl)imidazolidin-2-on
- 1,2-Dihydro-1-phenyl-3H-1,2,4-triazol-3-on
- Dichlorisocyanursäure
- 2-Cyan-N-((ethylamino)carbonyl)-2-(methoxyimino)acetamid
- 1,5-Dihydropyrimido[5,4-d]pyrimidin-2,4,6,8(3H,7H)tetron
- Hydrogenperoxid-Harnstoff
- 5-(sec-Pentyl)barbitursure
- Amino moulding plastic
1of8
Harnstoff Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(793)Suppliers
Region
-
Jiangsu Boquan Biotechnology Co., Ltd.
Telefon +86-18168774353
E-Mail boquanshengwu003@boquansw.com
-
Henan Bao Enluo International TradeCo.,LTD
Telefon +86-17331933971<br/>+86-17331933971
E-Mail deasea125996@gmail.com
-
Telefon
E-Mail tp@aladdinsci.com
-
Telefon
E-Mail tp@aladdinsci.com
-
Hebei Kingfiner Technology Development Co.Ltd
Telefon +86-15532196582<br/>+86-15373005021
E-Mail lisa@kingfinertech.com
-
Hebei Jingbo New Material Technology Co., Ltd
Telefon +8619931165850
E-Mail hbjbtech@163.com
-
Henan Fengda Chemical Co., Ltd
Telefon +86-371-86557731<br/>+86-13613820652
E-Mail info@fdachem.com
-
Hebei Kangcang new material Technology Co., LTD
Telefon +8615713292910
E-Mail Nancy@kangcang.com.cn
-
Yujiang Chemical (Shandong) Co.,Ltd.
Telefon +8617736087130
E-Mail catherine@yjchem.com.cn
-
Telefon +8619933239880
E-Mail admin@apcl.com.cn
1of2
57-13-6, Urea Verwandte Suche:
- Carbonylsulfid
- Hydrazin
- N,N-methylenebis N'-1-(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl urea
- Pyrazophos (ISO)
- Harnstoffphosphat
- Urease
- 1-Methyldiazolidin-2,4-dion
- 1-(((5-Nitro-2-furanyl)methylen)-amino)-2,4-imidazolidindion
- Dinatrium-3-[(4-acetamidophenyl)azo]-4-hydroxy-7-[[[[5-hydroxy-6-(phenylazo)-7-sulfonato-2-naphthyl]amino]carbonyl]amino]naphthalin-2-sulfonat
- 7,8-dimethylbenzo[g]pteridin-2,4(1H,3H)-dion
1of4
