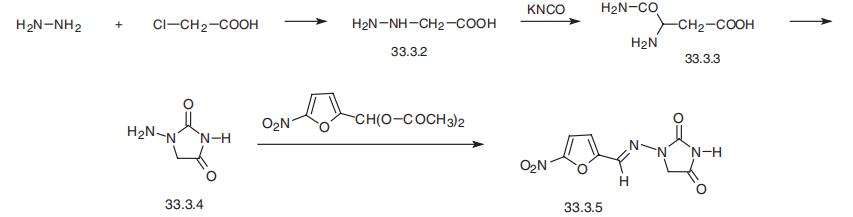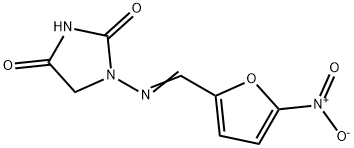
1-(((5-Nitro-2-furanyl)methylen)-amino)-2,4-imidazolidindion
Bezeichnung:1-(((5-Nitro-2-furanyl)methylen)-amino)-2,4-imidazolidindion
CAS-Nr67-20-9
Englisch Name:Nitrofurantoin
CBNumberCB7346080
SummenformelC8H6N4O5
Molgewicht238.16
MOL-Datei67-20-9.mol
Synonyma
Nitrofurantoin
1-(((5-Nitro-2-furanyl)methylen)-amino)-2,4-imidazolidindion
1-(((5-Nitro-2-furanyl)methylen)-amino)-2,4-imidazolidindion physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 268°C |
| Siedepunkt | 380.75°C (rough estimate) |
| Dichte | 1.5824 (rough estimate) |
| Brechungsindex | 1.6700 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| Löslichkeit | DMF: soluble50mg/mL |
| pka | 7.2(at 25℃) |
| Aggregatzustand | crystalline |
| Farbe | yellow |
| Wasserlöslichkeit | <0.01 g/100 mL at 19 ºC |
| Sensitive | Light Sensitive & Hygroscopic |
| maximale Wellenlänge (λmax) | 358nm(MeOH)(lit.) |
| Merck | 14,6599 |
| BRN | 893207 |
| BCS Class | 2 |
| Stabilität | Stability Stable, but light-sensitive. Combustible. Incompatible with strong oxidizing agents, strong alkalies, strong acids. Decomposes upon contact with most metals other than stainless steel and aluminium. |
| CAS Datenbank | 67-20-9(CAS DataBase Reference) |
| IARC | 3 (Vol. 50) 1990 |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 22-42/43 |
| S-Sätze: | 22-36/37-45 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| RTECS-Nr. | MU2800000 |
| TSCA | Yes |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29349990 |
| Giftige Stoffe Daten | 67-20-9(Hazardous Substances Data) |
| Toxizität | LD50 oral in rat: 604mg/kg |