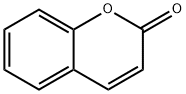
1-Benzopyran-2-on
Bezeichnung:1-Benzopyran-2-on
CAS-Nr91-64-5
Englisch Name:Coumarin
CBNumberCB3112168
SummenformelC9H6O2
Molgewicht146.14
MOL-Datei91-64-5.mol
Synonyma
Cumarin
1-Benzopyran-2-on
1,2-Benzopyron
1-Benzopyran-2-on physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 68-73 °C (lit.) |
| Siedepunkt | 298 °C (lit.) |
| Dichte | 0.935 |
| Dampfdruck | 0.01 mm Hg ( 47 °C) |
| Brechungsindex | 1.5100 (estimate) |
| Flammpunkt | 162 °C |
| storage temp. | Store below +30°C. |
| Löslichkeit | 1.7g/l |
| Aggregatzustand | Crystals or Crystalline Powder |
| Farbe | White |
| Säure-Base-Indikators(pH-Indikatoren) | Non' uorescence (9.5) to light green ' uorescence (10.5) |
| Geruch (Odor) | at 10.00 % in dipropylene glycol. sweet hay tonka new mown hay |
| Geruchsart | tonka |
| Biologische Quelle | synthetic |
| Wasserlöslichkeit | 1.7 g/L (20 ºC) |
| maximale Wellenlänge (λmax) | 275nm |
| Merck | 14,2562 |
| BRN | 383644 |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 22-40-36/37/38-20/21/22-43 |
| S-Sätze: | 36-36/37-26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 1 |
| RTECS-Nr. | GN4200000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29322010 |
| Giftige Stoffe Daten | 91-64-5(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats, guinea pigs: 680, 202 mg/kg (Jenner) |

