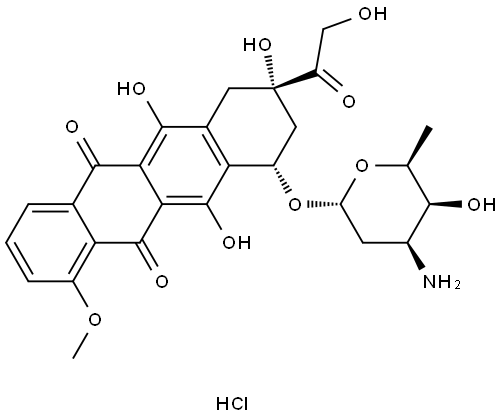
Doxorubicin hydrochloride
Bezeichnung:Doxorubicin hydrochloride
CAS-Nr25316-40-9
Englisch Name:Doxorubicin hydrochloride
CBNumberCB0110633
SummenformelC27H29NO11.ClH
Molgewicht579.98
MOL-Datei25316-40-9.mol
Synonyma
(8S-cis)-10-[(3-Amino-2,3,6-tridesoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxynaphthacen-5,12-dionhydrochlorid
Doxorubicin hydrochloride physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 216 °C (dec.)(lit.) |
| alpha | D20 +248±2° (c = 0.1 in methanol) |
| storage temp. | 2-8°C |
| Löslichkeit | Soluble in DMSO at 100 mg/ml; very poorly soluble in ethanol; soluble in water at 10 mg/ml with slight warming. |
| pka | pKa 8.25±0.60 (Uncertain);8.43±0.70 (Uncertain);11.9±0.4 (Uncertain);12.95±0.1 (Uncertain);13.8±0.70 (Uncertain) |
| Aggregatzustand | solid |
| Farbe | orange to dark red |
| Biologische Quelle | synthetic (organic) |
| Wasserlöslichkeit | Soluble in water. |
| maximale Wellenlänge (λmax) | 497nm(H2O)(lit.) |
| Merck | 14,3439 |
| BRN | 4229251 |
| Stabilität | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. |
| InChIKey | MWWSFMDVAYGXBV-RUELKSSGSA-N |
| CAS Datenbank | 25316-40-9(CAS DataBase Reference) |
| EPA chemische Informationen | Doxorubicin hydrochloride (25316-40-9) |
| Kennzeichnung gefährlicher | T,T+,Xi |
| R-Sätze: | 45-22-40-26/27/28-36/38 |
| S-Sätze: | 53-45-36/37/39-22-7/9 |
| WGK Germany | 3 |
| RTECS-Nr. | QI9295900 |
| F | 10-21 |
| HS Code | 29419090 |
| Toxizität | LD50 i.v. in mice: 21.1 mg/kg (Bertazzoli, 1970) |


