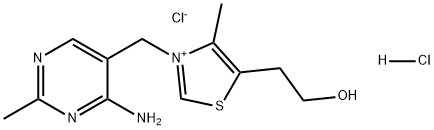
Description
Thiamine Hydrochloride(67-03-8) is the hydrochloride salt form of thiamine (vitamin B1), a vitamin essential for aerobic metabolism, cell growth, transmission of nerve impulses and acetylcholine synthesis. Vitamin B1 helps prevent various health problems including heart damage.
Thiamine hydrochloride is used to prevent and treat thiamine deficiency states, which may occur as a result of inadequate nutrition or intestinal malabsorption. It is also used for the treatment of Wernicke-Korsakoff syndrome, beriberi and thiamine deficiency related to chronic alcoholism. Thiamine hydrochloride is used as a food additive to add brothy/meaty flavor to gravies or soups. It is used also as a food supplement and flavoring ingredient with a bitter taste.
Chemical Properties
Thiamine hydrochloride, also known as vitamin B1, is a white or almost white, crystalline powder or colorless crystals. It has an odor slightly reminiscent of thiazole and a bitter taste. Very soluble in water (1g dissolved in 1mL of water at 20℃), slightly soluble in ethanol, and insoluble in ether, benzene, chloroform, and acetone. The oxidation-reduction reaction can cause it to lose activity. When exposed to air, the vitamin rapidly absorbs about 4% of water.
Occurrence
Rice husks are reportedly the principal source of vitamin B1; in variable amounts it is a constituent of yeast,
milk, green leaves, roots and tubers; it is also present in high concentration in seeds, and in lesser amount in different animal organs
and muscles.
History
Thiamine hydrochloride was the first water-soluble vitamin to be purified. In 1912, Cashmir Funk isolated thiamine from rice husks and coined the term ‘vitamine’ because it was required for life (‘vita’) and because thiamine contained nitrogen (‘amine’). Thiamine, formerly known as B1, is water soluble. Thiamine is a vital cofactor for enzymes and coenzymes of glycolysis, the Kreb’s cycle, the pentose phosphate pathway. Thiamine is also involved in the biosynthesis of the neurotransmitters acetylcholine and gamma-aminobutyric acid and in nerve propagation.
Uses
Thiamine(67-03-8) is the water-soluble vitamin b1, required for normal digestion and functioning of nerve tissues and in the prevention of beriberi. It also acts as a coenzyme in the metabolism of carbohydrates. During processing, the higher and longer the heating period, the greater the loss. The loss is reduced in the presence of acid. Thiamine hydrochloride and thiamine mononitrate are two available forms. The mononitrate form is less hygroscopic and more stable than the hydrochloride form, making it suitable for use in beverage powders. It is used in enriched flour and is found as thiamine mononitrite in frozen egg substitute and crackers.
Definition
ChEBI: A hydrochloride obtained by combining thiamine chloride with one molar equivalent of hydrochloric acid.
Preparation
By linking the preformed thiazole and pyrimidine ring system.
benefits
Thiamine is a essential nutrient required for carbohydrate metabolism; also involved in nerve function. Biosynthesized by microorganisms and plants. Dietary sources include whole grains, meat products , vegetables, milk, legumes and fruit. Also present in rice husks and yeast. Converted in vivo to Thiamine diphosphate, a coenzyme in the decarboxylation of α-keto acids. Chronic deficiency may lead t o neurological impairment, bariberi, Wernicke-Korsakoff syndrome. Thiamine hydrochloride is commonly used in the prevention and treatment of foot fungus caused by the lack of VB1, also used in neuritis, dyspepsia and other conditions of adjuvant therapy.
General Description
Thiamine is a water-soluble vitamin of the B complex whose phosphate derivatives are involved in many cellular processes required for overall human health. Thiamine deficiency, often the result of impaired nutritional status associated with chronic diseases from alcoholism to HIV-AIDS, is monitored in patient whole blood samples by HPLC.
Health Hazard
Diseases and disorders resulting from a deficiency of thiamine include beriberi, opisthotonos (in birds), polyneuritis, hyperesthesia, bradycardia, and edema. Rather than a specific disease, beriberi may be described as a clinical state resulting from a thiamine deficiency. In body cells, thiamine pyrophosphate is required for removing carbon dioxide from various substances, including pyruvic acid.
Biochem/physiol Actions
Thiamine(67-03-8) is an essential coenzyme in carbohydrate metabolism. Deficiency of thiamine causes beriberi, a neurological and cardiovascular disease. Thiamine is administered in case of deficiency, either due to reduced intake or synthesis. Congenital defect in the thiamine transporter gene SLC19A2 causes thiamine-responsive megaloblastic anemia syndrome (TRMA). Thiamine mimics acetylcholine in brain and possible exerts a role in Alzheimer′s disease. Thiamine deficiency in ruminants causes polioencephalomalacia.
Synthesis
General procedure for the synthesis of thiamine hydrochloride from the compound (CAS: 52164-53-1): 50 g of thiophene thiamine was added to a three-necked flask, 60 g of 31% hydrochloric acid solution was added, followed by cooling of the reaction system to -10°C in a freezer tank. Under strict temperature control at -30 °C, 30 g of 20% hydrogen peroxide solution was slowly added dropwise. After the dropwise addition was completed, the reaction mixture was held at -10°C for 20 hours. After completion of the reaction, the temperature was kept below 0°C and the filtration operation was carried out. The filter cake was washed with 50 g of methanol and dried to give 48.1 g of thiazole chloride hydrochloride in 94.7% yield. The product was quality analyzed and the result was satisfactory.
Environmental Fate
Thiamine and its common phosphate analogs are readily
soluble in water and ubiquitously utilized in nature.
Purification Methods
The hydrochloride crystallises from 95% EtOH (solubility is ca 1%). The monohydrate is dehydrated at 100o in vacuo over H2SO4, but is hygroscopic and picks up one molecule of H2O readily. It can be sterilised at 100o if the pH of the solution is below 5.5. The nitrate has m 196-200o(dec) and is more stable than the hydrochloride. The picrolonate crystallises from H2O and is dimorphic, m 164-165o and 228-229o(dec). [Todd & Bergel J Chem Soc 364, 367 1937, J Am Chem Soc 58 1063, 1504 1936, 59 526 1937, Beilstein 27 IV 1766.]
References
[1] https://www.drugs.com
[2] Shmuel Yannai (2012) Dictionary of Food Compounds with CD-ROM, Second Edition
[3] https://www.medicines.org.uk
[4] Fenaroli's Handbook of Flavor