Description
Ketoconazole (65277-42-1) is?a broad spectrum antifungal agent which acts via inhibition of a cytochrome P450, CYP51A1 which is a lanosterol 14α-demethylase.1 Also inhibits CYP3A and CYP1A1.2 Inhibits adrenal steroidogenesis.3 Downregulates cholesterol synthesis in drug-tolerant human lung cancer cell lines.4 Blocks the biosynthesis of leukotrienes (LT) via inhibition of 5-lipoxygenase and dose dependently inhibits LT-mediated bronchoconstriction in guinea pigs.5
Originator
Nizoral,Janssen,US,1981
Definition
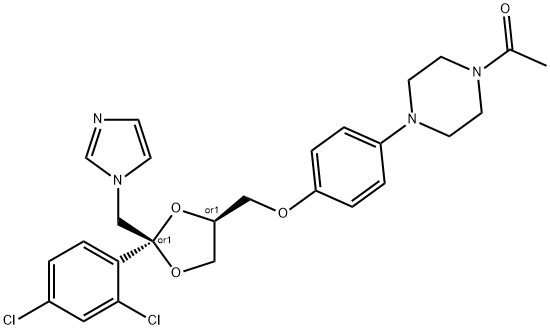
ChEBI: (2R,4S)-ketoconazole is a cis-1-acetyl-4-(4-{[2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazine which dioxolane moiety has (2R,4S)-configuration. It is an enantiomer of a (2S,4R)-ketoconazole.
Manufacturing Process
(A) A mixture of 33.8 parts of 4-(4-piperazinyl)phenol dihydrobromide, 11.2
parts of acetic acid anhydride, 42 parts of potassium carbonate and 300 partsof 1,4-dioxane is stirred and refluxed for 3 days. The reaction mixture is
filtered and the filtrate is evaporated. The solid residue is stirred in water and
sodium hydrogen carbonate is added. The whole is stirred for 30 minutes. The
precipitated product is filtered off and dissolved in a diluted hydrochloric acid
solution. The solution is extracted with trichloromethane. The acid aqueous
phase is separated and neutralized with ammonium hydroxide. The product is
filtered off and crystallized from ethanol, yielding 5.7 parts of 1-acetyl-4-(4-
hydroxyphenyl)piperazine; MP 181-183°C.
(B) A mixture of 2.4 parts of 1-acetyl-4-(4-hydroxyphenyl)piperazine, 0.4 part
of sodium hydride dispersion 78%; 75 parts of dimethylsulfoxide and 22.5
parts of benzene is stirred for one hour at 40°C. Then there are added 4.2
parts of cis-2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-
ylmethyl methane sulfonate and stirring is continued overnight at 100°C. The
reaction mixture is cooled and diluted with water. The product is extracted
with 1,1'-oxybisethane. The extract is dried, filtered and evaporated. The
residue is crystallized from 4-methyl-2-pentanone. The product is filtered off
and dried, yielding 3.2 parts (59%) of cis-1-acetyl-4-[2-(2,4-dichlorophenyl)-
2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-ylmethoxyl phenyl]piperazine; MP
146°C.
Brand name
Ketozole (Taro); Nizoral (Janssen); Nizoral (McNeil);Cerozalol;Cetonax;Fetonal;Fungarol;Fungo-hubber;Ketocidin;Ketoisdin;Ketonan;Ketoral;Micoral;Micotek;Micoticum;Nizcrem;Nizoral 2% shampoo;Nizoral 20% cream;Nizovules;Nizshampoo;Oromycosal;Oronazol;Panfungol;Rofenid;Spike;Unidox.
Therapeutic Function
Antifungal
World Health Organization (WHO)
Ketoconazole, an imidazole antifungal agent, was introduced in
1978 for the topical and systemic treatment of a wide variety of fungal infections.
Its use by mouth has been associated with hepatotoxicity, including cases of
hepatitis, which have usually been reversible on discontinuation of the drug, but
some fatalities have also occurred. Ketoconazole is widely marketed.
Antimicrobial activity
The spectrum includes dermatophytes, some dimorphic fungi
and Candida spp.
Acquired resistance
Resistance has been documented in patients treated for
chronic mucocutaneous candidosis and AIDS patients with
oropharyngeal or esophageal candidosis. Some fluconazoleresistant
C. albicans and C. glabrata are cross-resistant to
ketoconazole.
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Ketoconazole is an imidazole antifungal agent administered through topical or oral means. It is used for the treatment of chronic mucocutaneous candidiasis, fungal infections of the gastro-intestinal tract, dermatophyte infections, systemic infections, and fungal infections in immuno-compromised patients.
Pharmaceutical Applications
A synthetic dioxolane imidazole available for oral and topical
use.
Biological Activity
Antifungal agent; potent inhibitor of cytochrome P450c17.
Biochem/physiol Actions
Ketoconazole is an imidazole derivative. It plays an important role in inhibiting the conversion of lanosterol to ergosterol in the cell wall of fungi. Ketoconazole has therapeutic effects against dermatophytosis, superficial candidiasis, and paracoccidioidomycosis.
Mechanism of action
Ketoconazole has little effect on Aspergillus or Cryptococcus. Ketoconazole is highly dependent on low stomach pH for absorption, and antacids or drugs that raise stomach pH will lower the bioavailability of ketoconazole. As with other azoles, it is extensively metabolized by microsomal enzymes. All the metabolites are inactive. Evidence that CYP3A4 plays a significant role in metabolism of ketoconazole is that coadministration of CYP3A4 inducers, such as phenytoin, carbamazepine, and rifampin, can cause as much as a 50% reduction in levels of ketoconazole.
Clinical Use
Ketoconazole can be used as palliative treatment for
Cushing’s syndrome in patients undergoing surgery or
receiving pituitary radiation and in those for whom
more definitive treatment is still contemplated. Because
surgical treatment is not always well tolerated by elderly
patients, ketoconazole 200 to 1,000 mg/day can be a
valuable alternative for the control of hypercortisolism.
Common side effects include pruritus, liver dysfunction,
and gastrointestinal symptoms.
Clinical Use
Ketoconazole remains useful in the treatment of cutaneous
and mucous membrane dermatophyte and yeast
infections, but it has been replaced by the newer triazoles
in the treatment of most serious Candida infections
and disseminated mycoses. Ketoconazole is usually
effective in the treatment of thrush, but fluconazole
is superior to ketoconazole for refractory thrush.
Widespread dermatophyte infections on skin surfaces
can be treated easily with oral ketoconazole when the
use of topical antifungal agents would be impractical.
Treatment of vulvovaginal candidiasis with topical imidazoles
is less expensive.
Clinical Use
Mucosal candidosis
Pityriasis versicolor
Seborrheic dermatitis
Non-life-threatening forms of blastomycosis, coccidioidomycosis,
histoplasmosis and paracoccidioidomycosis
Synthesis
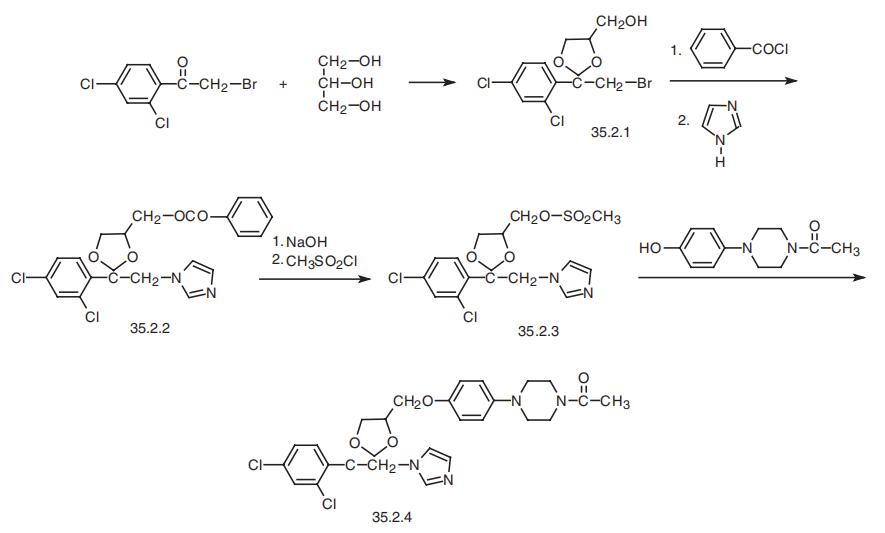
Ketoconazole, cis-1-acetyl-4-[4-[2-(2,4-dichlorophenyl)-2-(1H-imidazole-
1-ylmethyl)-1,3-dioxolan-4-ylmethyl]phenyl]piperazine (35.2.4), is synthesized from
2,4-dichlorophenacyl bromide, the ketalization of which using glycerol gives cis-2-(2,4-
dichlorophenyl)-2-bromoethyl-4-hydroxymethyl-1,3-dioxolane (35.2.1). Acylating the
hydroxyl group of this compound with benzoyl chloride, and then alkylating the resulting
compound with imidazole gives the derivative (35.2.2). Next, alkaline hydrolysis removes
the benzoyl group, and a reaction with methanesulfonyl chloride gives a mesylate (35.2.3).
Finally, alkylating the resulting 1-acetyl-4-(4-hydroxyphenyl)piperazine gives ketoconazole
(35.2.4).
Veterinary Drugs and Treatments
Because of its comparative lack of toxicity when compared to amphotericin
B, oral administration, and relatively good efficacy, ketoconazole
has been used to treat several fungal infections in dogs,
cats, and other small species. Ketoconazole is often employed with
amphotericin B to enhance the efficacy of ketoconazole, and by
reducing the dose of amphotericin B, decreasing its risk of toxicity.
See the Dosage section or Pharmacology section for specifics.
Newer antifungal agents (fluconazole, itraconazole) have advantages
over ketoconazole, primarily less toxicity and/or enhanced
efficacy; however, ketoconazole can be significantly less expensive
than the newer agents. Ketoconazole is considered by some to still
be the drug of choice for treating histoplasmosis in dogs.
Use of ketoconazole in cats is controversial and some say it should
never be used that species.
Ketoconazole is also used clinically for the medical treatment of
hyperadrenocorticism in dogs. Ketoconazole
appears to be a viable
option (although relatively expensive) to mitotane, particularly for
palliative
therapy in dogs with large, malignant, or invasive tumors
where surgery is not an option. Ketoconazole is also used frequently
in dogs for stabilization prior to surgery. It is a reversible inhibitor
of steroidogenesis, so it is usually not a viable option for long-term
treatment.
Because it interferes with the metabolism of cyclosporine, it has
been used to reduce the dosage necessary for cyclosporine in dogs.
Drug interactions
Potentially hazardous interactions with other drugs
Aminophylline and theophylline; possibly increased
concentration of aminophylline and theophylline.
Analgesics: inhibits buprenorphine metabolism -
reduce buprenorphine dose; possible increased risk
of ventricular arrhythmias with methadone - avoid;
increases oxycodone and sufentanil concentration;
avoid with paracetamol.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with disopyramide - avoid;
concentration of dronedarone increased - avoid.
Antibacterials: metabolism increased by rifampicin;
may reduce rifampicin concentration; concentration
of bedaquiline increased - avoid; avoid with
fidaxomicin; concentration possibly reduced
by isoniazid; avoid with clarithromycin and
telithromycin in severe renal (both) and hepatic
impairment (telithromycin only).
Anticoagulants: anticoagulant effect of coumarins
enhanced; concentration of apixaban, dabigatran
and rivaroxaban increased - avoid; concentration of
edoxaban increased - reduce edoxaban dose.
Antidepressants: avoid concomitant use with
reboxetine; ketoconazole increases concentration of
mirtazapine.
Antidiabetics: concentration of pioglitazone,
saxagliptin and tolbutamide increased.
Antiepileptics: concentration of ketoconazole
reduced by fosphenytoin and phenytoin and possibly
carbamazepine; concentration of perampanel and
possibly carbamazepine increased.
Antifungals: concentration of isavuconazole
increased - avoid.
Antihistamines: concentration of loratidine
possibly increased - avoid; avoid with mizolastine;
concentration of rupatadine increased.
Antimalarials: avoid with piperaquine with artenimol
and artemether and lumefantrine; concentration of
mefloquine increased.
Antimuscarinics: absorption of ketoconazole
reduced; concentration of darifenacin increased -
avoid; reduce dose of fesoterodine; concentration
of oxybutynin and solifenacin increased; avoid with
tolterodine.
Antipsychotics: increased risk of ventricular
arrhythmias with pimozide - avoid; possibly
increased concentration of quetiapine - reduce
quetiapine dose; inhibits aripiprazole metabolism -
reduce aripiprazole dose; concentration of lurasidone
increased - avoid
Metabolism
Ketoconazole is extensively degraded by the liver, and very little active
drug is excreted in either the urine or bile; the dose need not be modified
for renal insufficiency. Adverse reactions to topical ketoconazole are very
rare.
storage
Desiccate at +4°C
References
1) Lambert et al. (1986) The effects if ketoconazole on adrenal and testicular steroidogenesis in vitro; Biochem. Pharmacol. 35 3999
2) Sai et al. (2000) Assessment of specificity of eight chemical inhibitors using cDNA-expressed cytochromes P450. Xenobiotica 30 327
3) Loose et al. (1983) Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes; J. Clin. Invest. 71 1495
4) Howell et al. (2019) Lung cancer cells survive epidermal growth factor receptor tyrosine kinase inhibitor exposure through upregulation of cholesterol synthesis; FASEB Bioadv. 2 90
5) Beetens et al. (1986) Ketoconazole inhibits the biosynthesis of leukotrienes in vitro and in vivo; Biochem. Pharmacol. 35 883