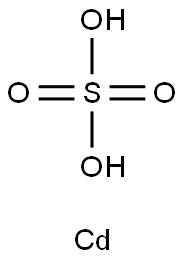
General Description
Odorless white solid. Sinks and mixes slowly with water.
Reactivity Profile
CADMIUM SULFATE(10124-36-4) acts as a weakly acidic inorganic salt, which is soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Air & Water Reactions
Water soluble.
Hazard
A confirmed carcinogen.
Health Hazard
Exposures to cadmium salts by absorption are most effi cient via the respiratory tract. The
symptoms of health effects include, but are not limited to, irritation, headache, metallic
taste, and/or cough. Severe exposures cause shortness of breath, chest pain, and fl u-like
symptoms with weakness, fever, headache, chills, sweating, nausea and muscular pain,
pulmonary edema, liver and kidney damage, and death. Prolonged exposures, even at
relatively low concentrations, may result in kidney damage, anemia, pulmonary fi brosis,
emphysema, perforation of the nasal septum, loss of smell, male reproductive effects, and
an increased risk of cancer of the lung and of the prostate. Decrease in bone density, renal
stones, and other evidence of disturbed calcium metabolism have been reported.
Health Hazard
Inhalation may cause dryness of throat, coughing, constriction in chest, and headache. Ingestion may cause salivation, vomiting, abdominal pains, or diarrhea. Contact with eyes causes irritation.
Potential Exposure
It is used in pigments, electroplating;
as a fungicide; and in synthetic and analytical chemistry.
Also used in fluorescent screens; as an electrolyte.
Incompatibilities: Acts as a weak inorganic acid; neutralizes bases. Incompatible with oxidizers (chlorates, nitrates,
peroxides, permanganates, perchlorates, chlorine, bromine,
fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides, sulfur, selenium, tellurium, zinc
Fire Hazard
Special Hazards of Combustion Products: Toxic cadmium oxide fume may form in fires.
First aid
Move victim to fresh air. Call 911 or emergency
medical service. Give artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; give artificial respiration with the aid of a pocket mask equipped with a one-way
valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running water for at least 20 minutes. For minor skin contact,
avoid spreading material on unaffected skin. Keep victim
warm and quiet. Effects of exposure (inhalation, ingestion,
or skin contact) to substance may be delayed. Ensure that
medical personnel are aware of the material(s) involved
and take precautions to protect themselves. Medical
Shipping
UN2570 Cadmium compounds, Hazard Class:
6.1; Labels: 6.1-Poisonous materials, Technical Name
Required.
Description
Cadmium sulfate is a white to colorless, odorless, crystalline substance. Molecular weight=208.48;Freezing/Melting point=1000℃. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 0, Reactivity 0. Soluble in water;solubility=76 g/100 mL at 0℃.
Chemical Properties
Cadmium sulfate is a white to colorless, odorless, crystalline substance.
Chemical Properties
Cadmiumsulfate, CdS04, is an effiorescent crystalline solid that is soluble in water. It is used as an antiseptic,in the treatment of venereal diseases and rheumatism, and to detect the presence of hydrogen sulfide.
Physical properties
Colorless orthogonal crystal; the hydrates have monoclinic crystal system; density 4.69 g/cm3 (density of mono-, and octahydrates is 3.79 and 3.08 g/cm3, respectively); melts at 1,000°C (octahydrate decomposes at 40°C); soluble in water, insoluble in ethanol.
Definition
ChEBI: Cadmium sulfate is a cadmium salt.
Preparation
Cadmium sulfate is prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid:
CdO + H2SO4 → CdSO4+ H2
CdO + H2SO4 → CdSO4 + H2O
Cd(OH)2 + H2SO4 → CdSO4+ 2H2O.
reaction suitability
reagent type: catalyst
core: cadmium
storage
Color Code—Blue: Health Hazard: Store in asecure poison location. Prior to working with Cadmium sulfate, you should be trained on its proper handling and storage. A regulated, marked area should be established wherethis chemical is handled, used, or stored in compliance withOSHA Standard 1910.1045. Store in tightly closed containers in a cool, well-ventilated area away from oxidizers andmetals.
Incompatibilities
Incompatible with strong oxidizers,sulfur, selenium, tellurium, zinc. Acts as a weak inorganicacid; neutralizes bases. May ignite combustible materials.