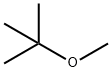
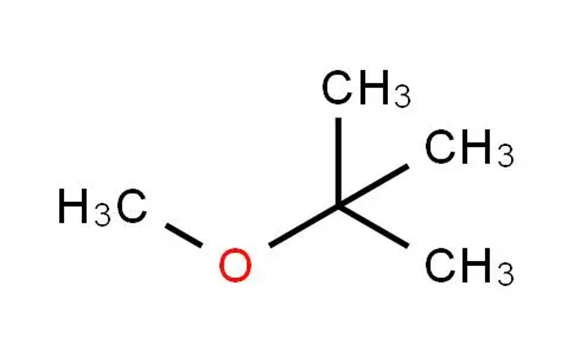
What is Methyl tert-butyl ether?
Methyl tert-butyl ether is an ether having methyl and tert-butyl as the two alkyl components. It has a role as a non-polar solvent, a fuel additive and a metabolite. Methyl tert-butyl ether appears as a colorless liquid with a distinctive anesthetic-like odor. Vapors are heavier than air and narcotic. Boiling point 131°F. Flash point 18°F. Less dense than water and miscible in water. Used as a octane booster in gasoline.
Uses
tert-Butyl methyl ether is a commonly used organic solvent that can be synthesized by acid catalyzed reaction between methanol and isobutene. It is an effective alternative to lead containing additives for enhancing the octane rating of gasoline.
Methyl tert-butyl ether is used as a gasoline additive. Exposure may occur by breathing air contaminated with auto exhaust or gasoline fumes while refueling autos. Respiratory irritation, dizziness, and disorientation have been reported by some motorists and occupationally exposed workers. Acute (short- term) exposure of humans to methyl tert-butyl ether also has occurred during its use as a medical treatment to dissolve cholesterol gallstones. Chronic (long-term) inhalation exposure to methyl tert-butyl ether has resulted in central nervous system (CNS) effects, respiratory irritation, liver and kidney effects, and decreased body weight gain in animals. Developmental effects have been reported in rats and mice exposed via inhalation. EPA has not classified methyltert-butyl ether with respect to potential carcinogenicity.
In the U.S. MTBE has been used in gasoline at low levels since 1979, replacing tetraethyllead (TEL) as an antiknock (octane rating) additive to prevent engine knocking. Oxygenates also help gasoline burn more completely, reducing tailpipe emissions and dilute or displace gasoline components such as aromatics (e.g., benzene). Before the introduction of other oxygenates and octane enhancers, refiners chose MTBE for its blending characteristics and low cost.
MTBE forms azeotropes with water (52.6 °C; 96.5% MTBE) and methanol (51.3 °C; 68.6% MTBE).
In a medical procedure called contact dissolution therapy, MTBE is injected directly into the gallbladder to dissolve gallstones.
MTBE undergoes oxidative degradation in the presence of propane-oxidizing bacterial strains. The kinetic studies of heat-assisted persulfate oxidation of MTBE under various parameters suggests that the reaction follows the pseudo-first-order kinetics. MTBE can be synthesized by acid catalyzed reaction between methanol and isobutene. A study suggests that the addition of MTBE increases the number of active sites during polymerization of propene by stopped-flow method.
Solvent Application
MTBE is extensively used in industry as a safer alternative to diethyl ether (which is commonly used in academic research) as the tert-butyl group prevents MTBE from forming potentially explosive peroxides. It also is used as a solvent in academic research, although it is used less commonly than diethyl ether. Although an ether, MTBE is a poor Lewis base and does not support formation of Grignard reagents. It is also unstable toward strong acids. It reacts dangerously with bromine.
As a solvent, tert-Butyl methyl ether may be used to synthesize fatty acid methyl esters (FAMEs) and glycerol tert-butyl ether via transesterification with canola oil under supercritical conditions.
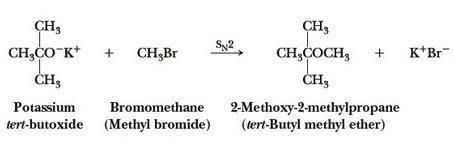
Preparation
tert-butyl methyl ether can be prepared by the reaction of potassium tert-butoxide and bromomethane.
Health Hazard
INHALATION: May cause dizziness or suffocation. Contact may irritate or burn eyes or skin. May be harmful if swallowed.
Carcinogenicity
Basis of determination of carcinogenicity of chemical compounds. There is general agreement among experts in chemical carcinogenesis that a substance that causes cancer in significant numbers of experimental animals in well-conducted assays poses a presumptive carcinogenic risk to humans, even in the absence of confirmatory epidemiological data. This principle is accepted by scientific and medical experts throughout the world and has served for many years as the basis for sound public health policy and regulatory action on carcinogens. For example, the International Agency for Research on Cancer (IARC) of the World Health Organization in its Supplement 7 of the Monograph states.
Ntp criteria for listing chemicals as “reasonably anticipated to be a human carcinogen”.
Evidence of MTBE as a potential human carcinogen. Evidence from three separate animal bioassay studies (two different species of rats and in mice) demonstrates that chronic exposure to MTBE by either oral or inhalation route of exposure causes cancers in animals.
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butyl methyl ether manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1634-04-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 1634-04-4
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons