Introduction of Acetylacetone
General description
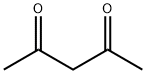
Acetylacetone is a beta-diketone that is pentane in which the hydrogens at positions 2 and 4 are replaced by oxo groups. It is a conjugate acid of an acetylacetonate. Pentane-2,4-dione appears as a colorless or yellow colored liquid. Less dense than water. Vapors are heavier than air. Used as a solvent in paints and varnishes.[1]
Application
Acetylacetone, also known as 2,4-pentanedione, is an important commodity chemical and widely used as a fuel additive, as dyeing intermediate, in the fields of metal extraction, metal plating, and resin modification. Hantzsch[2] reaction was used as a derivatizing agent for the assay of compounds having a primary amino group. The reagent was reacted with the primary amino group of the drugs to form a product having color and/or emit fluorescence. This condensation reaction was distinguished by its precision, reproducibility, and analytical cost reduction. FLX contains an aliphatic amino group, in the presence of formaldehyde solution, this amino group can condense with two equivalents of acetylacetone to form dihydropyridine derivative that emits yellow fluorescent product. (Figure1). Under optimized conditions of the reaction, FLX gave highly fluorescent product measured at λem 479 nm using 419 nm as excitation.
Figure 1. Proposed pathway of the Hantzsch reaction between FLX, acetylacetone and formaldehyde
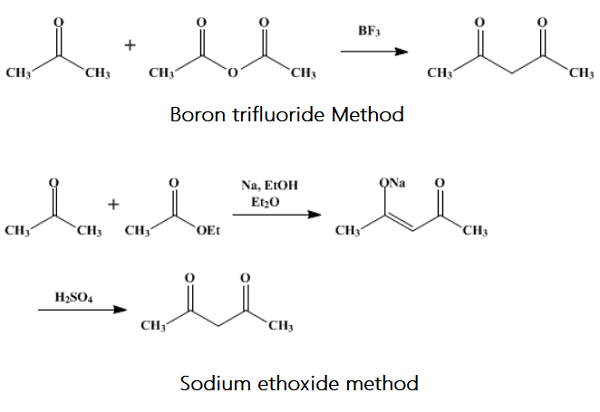
Synthesis
Acetylacetone is a commercially bulk chemical with diverse applications. However, the traditional manufacturing methods suffer from many drawbacks such as multiple steps, harsh conditions, low yield, and environmental problems, which hamper further applications of petrochemical-based acetylacetone. Compared to conventional chemical methods, biosynthetic methods possess advantages such as being eco-friendly, and having mild conditions, high selectivity and low potential costs. It is urgent to develop biosynthetic route for acetylacetone to avoid the present problems. Traditionally, acetylacetone is manufactured through chemical routes using acetone and ketene, which is produced by pyrolysis of acetone or acetic acid at a temperature of 700–800 °C, with carbon monoxide, methane, hydrogen formed as by-products In specific, esterification of ketene and acetone forms isopropenyl acetate (IPA), in the presence of a strong acid catalyst. Then, IPA is transformed into acetylacetone at 500–600 °C with metallic molybdenum as a catalyst, whereby the yield is only about 45%. In conclusion, the chemical routes suffer from drawbacks such as multiple steps, harsh conditions, low yield, and environmental problems, which hamper further applications of petrochemical-based acetylacetone. To address the issue, new methods need to be developed for acetylacetone preparation. Compared to conventional chemical methods, bio-synthetic methods are expected to have advantages such as being eco-friendly and having mild conditions.
The biosynthetic pathway of acetylacetone was constructed by reversing its biodegradation route, and the acetylacetone was successfully produced by engineered Escherichia coli (E. coli) by overexpression of acetylacetone cleaving enzyme (Dke1) from Acinetobacter johnsonii. Several promising amino acid residues were selected for enzyme improvement based on sequence alignment and structure analysis, and the acetylacetone production was improved by site-directed mutagenesis of Dke1. The double-mutant (K15Q/A60D) strain presented the highest acetylacetone-producing capacity which is 3.6-fold higher than that of the wild-type protein. Finally, the strain accumulated 556.3 ± 15.2 mg/L acetylacetone in fed-batch fermentation under anaerobic conditions.[3]
Toxicity
Moderate toxicity, can stimulate skin and mucous membrane. If the human body stays at 150 ~ 300mg / kg for a long time, it will have symptoms such as headache, nausea, vomiting, vertigo and sensory retardation.
Storage
Its vapor and air can form an explosive mixture, which can cause combustion and explosion in case of open fire and high heat. It can react with oxidant. If the flow rate is too fast, it is easy to generate and accumulate static electricity. Its vapor is heavier than air and can diffuse to a considerable distance at a lower place. It will catch fire and burn back in case of fire source. In case of high heat, the internal pressure of the container will increase, and there is a risk of cracking and explosion.
Reference
1.Jin J., Zhang S. & Wu B. et al., "Enhanced Photooxidation of Hydroquinone by Acetylacetone, a Novel Photosensitizer and Electron Shuttle," Environmental Science & Technology, Vol.53, No.19(2019), pp.11232-11239.
2.Abu Hassan A. A., Omar M. A. & Derayea S. M., "Use of acetylacetone for nano‐level assay of fluvoxamine maleate in pure form and pharmaceutical formulation," Luminescence, Vol.35, No.8(2020), pp.1360-1365.
3.Zhou Y., Ding Y. & Gao W. et al., "Biosynthesis of acetylacetone inspired by its biodegradation," Biotechnology for Biofuels, Vol.13, No.1(2020).
You may like
Related articles And Qustion
Lastest Price from Acetylacetone manufacturers

US $1.00/kg2025-04-21
- CAS:
- 123-54-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 123-54-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons