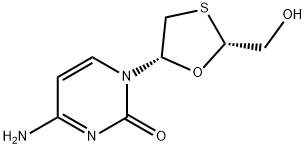
Lamivudine for the treatment of HIV
General description
Lamivudine is a nucleoside analogue and reverse transcriptase inhibitor used in the therapy of human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infection. Lamivudine is a very rare cause of clinically apparent drug induced liver injury, but is associated with flares of underlying hepatitis B during therapy or with abrupt withdrawal. Lamivudine (formerly known as 3TC) in combination with zidovudine is indicated for the treatment of HIV infection in patients who experience disease progression as evidenced by clinical and/or immunological test.
Application and pharmacology
Lamivudine is a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Intracellularly, lamivudine is phosphorylated to its active metabolites, lamiduvine triphosphate (L-TP) and lamiduvine monophosphate (L-MP). In HIV, L-TP inhibits HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation of the nucleoside analogue into viral DNA. In HBV, incorporation of L-MP into viral DNA by HBV polymerase results in DNA chain termination. L-TP is a weak inhibitor of mammalian DNA polymerases alpha and beta, and mitochondrial DNA polymerase. (NCI04) It is a monothioacetal that consists of cytosine having a (2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl moiety attached at position 1. An inhibitor of HIV-1 reverse transcriptase, it is used as an antiviral in the treatment of AIDS and hepatitis B. It has a role as a HIV-1 reverse transcriptase inhibitor, an antiviral drug, an anti-HBV agent, an allergen, a prodrug and an EC 2.7.7.49 (RNA-directed DNA polymerase) inhibitor. It is a monothioacetal, a primary alcohol, an oxacycle and a nucleoside analogue. It derives from a cytosine.[1,2]
Synthesis
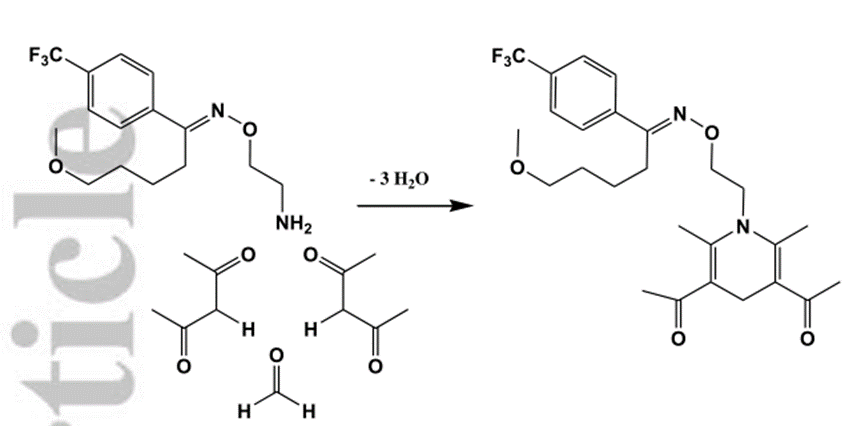
At the beginning of the design, it was intended to follow the principle of simplicity and convenience to splice the two through one-step reaction. However, through repeated experiments, it was found that due to the poor solubility of the two raw materials in most organic solvents and the mutual interference of active functional groups such as amino and hydroxyl groups, it was difficult to separate and purify the target product from the reaction solution or no reaction occurred. At the same time, the effect of activating carboxyl group by acyl chloride is not ideal. Therefore, the experimental scheme of protecting other active functional groups, then conducting synthetic reaction, and finally removing protective groups is adopted. For the amidation reaction, the 5 '- hydroxyl group of lamivudine and the two phenolic hydroxyl groups at the 3 - and 4-positions of protocatechuic acid are the protection objects, and the same protective group R1 is used for later removal. The steps of removing the protective group are carried out according to the common steps. The design synthesis route of prodrug 2-2 is shown in Figure 1. The other part of the synthesis route we can find in the reference.[3]
Figure1 Design and synthesis of the prodrugs of Lamivudine
History
Lamivudine in combination with other NRTIs and either a PI or an NNRTI has remained a backbone agent of choice since its initial approval in 1995 based on its established efficacy and favorable safety profile in a wide variety of patient populations. Lamivudine is considered one of the best-tolerated agents among all antiretroviral classes and has been a main component of combination ART of pivotal registration trials for new agents in existing or novel classes of therapy including PIs, NNRTIs, CCR5 antagonists and integrase inhibitors. Compared to other NRTIs, lamivudine is not associated with anemia (as is zidovudine) or nephrotoxicity (as is tenofovir). It has better gastrointestinal tolerability and less potential for decreased bone density, dyslipidemia, fat redistribution or long-term mitochondrial toxicity resulting in lactic acidosis or hepatic steatosis compared to other NRTIs. It has utility for both initial ART as well as for later lines of therapy due to its ability to delay the emergence of zidovudine-resistant mutants or restore zidovudine sensitivity. The patient continues to receive benefit from lamivudine even after the emergence of lamivudine resistant HIV-1, due to the reduced fitness of the resistant virus. HIV containing M184V (the mutation selected by lamivudine) replicates less efficiently than does wild type virus, resulting in reduced plasma HIV RNA. In the absence of lamivudine, the wild type HIV becomes the majority species and the fitness benefit is lost.
Toxicity
Lamivudine is rapidly absorbed after oral consumption. Fasted and non-fasted states do not interfere with lamivudine absorption; therefore, lamivudine can be administered with or without food. Binding of lamivudine to plasma proteins is <36%. The primary route of excretion is in the urine. The mean elimination half-life ranges from 5 to 7 hours. Decreased renal function increases the mean elimination half-life of lamivudine. Pharmokinetics effects of impaired renal function in pediatric patients are not known. Pharmokinetic properties of lamivudine have not been studied with respect to race, gender, or geriatrics.
Reference
1.Kumar P. N. & Patel P., "Lamivudine for the treatment of HIV," Expert Opinion on Drug Metabolism & Toxicology, Vol.6, No.1(2009), pp.105-114.
2.Treatment Review
3.Chen Qi: design, synthesis and evaluation of anti HBV activity of lamivudine polyphenols: Hubei University, 2020.
You may like
Related articles And Qustion
Lastest Price from Lamivudine manufacturers
US $0.00/kg2025-11-27
- CAS:
- 134678-17-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 134678-17-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 500kg