Application of Dicyandiamide
General description
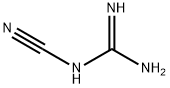
Dicyanodiammonia, abbreviated as dicy or DCD. It is an organic substance with the chemical formula of c2h4n4. It is a dimer of cyanamide and a cyano derivative of guanidine. Chemical formula c2h4n4. White crystalline powder. Soluble in water, alcohol, ethylene glycol and dimethylformamide, almost insoluble in ether and benzene. Stable when dry. It is a guanidine in which one of the amino hydrogens of guanidine itself is substituted by a cyano group. It is used in the manufacture of fertilizers, pharmaceuticals, explosives, oil well drilling muds, and dyestuffs. It has a role as a curing agent, a flame retardant, a fertilizer, an explosive and a nitrification inhibitor. It is a member of guanidines and a nitrile. The application of nitrification inhibitors has been used as a strategy to promote N utilization efficacy and reduce N2O emissions in paddy Dicyandiamide (DCD) as a widely used nitrification inhibitor inhibits the activity of ammonium-oxidizing bacteria which results in longer ammonium retention and reduces the production of NO2−in soils. DCD efficacy was found to be related to DCD concentration, temperature, moisture, pH, and organic matter content. Studies have shown that leaching DCD from agricultural soils into aquatic ecosystems can strongly change the community composition of benthic stream bacteria and algae and influence stream nutrient cycling stoichiometry. Literature on the mechanisms and benefits of nitrification inhibitors is extensive but there are very few studies focused on the influence of DCD application on other microbes in paddy system.
Application
1.Dicyandiamide (DCD) and thiosulfates are two type of nitrification inhibitors (NIs) that have been widely used in agriculture to improve nitrogen (N) fertilizer use efficiency and mitigate negative effect of N on environment. Little information is available concerning the comparison of the efficacy of DCD and thiosulfate on N transformations in soil. The aim of this study was to compare the effects of DCD and thiosulfate (K2S2O3) on changes of NH4+-N, nitrification inhibition and N recovery in a latosolic red soil. An incubation experiment was conducted with four treatments of control (CK), N, N+DCD, and N+K2S2O3. Soil samples were collected periodically over 50 d to determine concentrations of mineral N, and theamo A gene abundance of ammonia monooxygenase (AMO) for ammonia-oxidizing bacteria (AOB) wasestimated by qPCR after 10 d incubation. [1,2]
2.Dicyandiamide (DCD) is commonly used as nitrification inhibitors which has the potential to reduce nitrogen loss from paddy soils. In paddy systems, periphytic biofilms are commonly presented at the soil/water interface and show significant effects on nutrient cycling. However, the interaction between DCD and periphytic biofilms in paddy and subsequent effects on nitrogen cycling is unclear. In this work, microcosm experiments were carried out to study the interaction between the periphytic biofilms and DCD and the potential influence on nitrogen cycling from in paddy. Results showed that DCD affected the development of periphytic biofilms, while the presence of periphytic biofilms accelerated DCD degradation. Results also showed DCD application reduced nitrification potential mainly by inhibiting ammonia-oxidizing bacteria (AOB). Higher DCD dosage increased NH3volatilization loss. However, presence of periphytic biofilm reduced the NH3volatilization loss but increased denitrification. Our work contributes to a better understanding on the nitrogen cycling processes in paddy, and provides useful information for the improvement of nitrogen utilization efficiency and the control of non-point source pollution.[1]
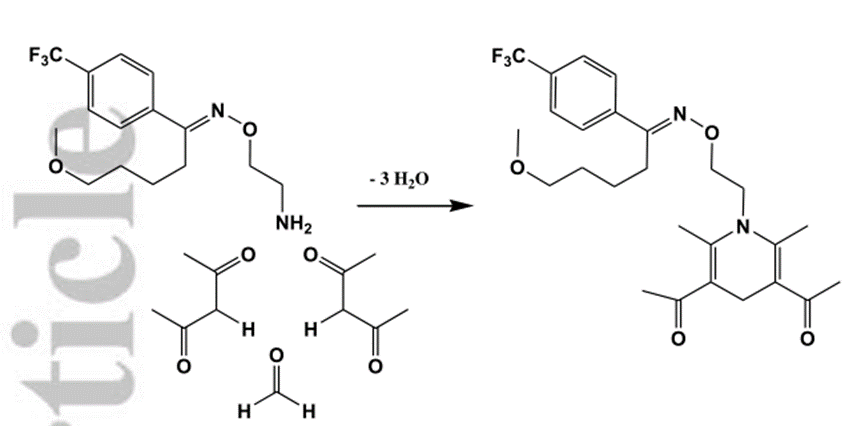
Figure1 the application of Dicyandiamide
3.As an epoxy resin curing agent with excellent latent performance, dicyandiamide is widely used in epoxy prepreg. The room temperature incubation period of the product after dicyandiamide and resin mixing is more than 6 months, and the cured product has excellent chemical stability, mechanical properties and electrical properties.[3]
Synthesis
The calcium hydrogen cyanide suspension obtained from the hydrolysis of calcium cyanamide is filtered under reduced pressure to remove the calcium hydroxide filter residue, and then carbon dioxide is introduced into the filtrate to precipitate the calcium in the form of calcium carbonate to obtain the cyanamide solution. It is polymerized under alkaline conditions, then filtered, cooled, crystallized, separated and dried to obtain dimer cyanamide. The temperature at the maximum rate of dicyandiamide formation is related to pH: pH is 9.7 at 50 ℃; PH 9.1 at 80 ℃; The pH is 8.8 at 100 ℃. After controlled polymerization under these conditions, the finished dicyandiamide is obtained by cooling, crystallization, separation and drying. The content of dicyandiamide in industrial products is 99%, and 4239kg of lime nitrogen (more than 21% nitrogen) is consumed per ton of products.
Reference
1.Zhao Y., Liu H. & Wang R. et al., "Interactions between dicyandiamide and periphytic biofilms in paddy soils and subsequent effects on nitrogen cycling," The Science of the total environment, Vol.718(2020), p.137417.
2.Ning J., Ai S. & Cui L., "Dicyandiamide has more inhibitory activities on nitrification than thiosulfate," PLOS ONE, Vol.13, No.8(2018), p.e200598.
3.Nanxun, Zhou Yu, Ling Hui, etc.: Research Progress of modified dicyandiamide, aerospace material technology, 2019, issue 02, page 7-10.
You may like
Related articles And Qustion
Lastest Price from Dicyandiamide manufacturers

US $0.00-0.00/kg2025-09-30
- CAS:
- 461-58-5
- Min. Order:
- 1000kg
- Purity:
- 0.99
- Supply Ability:
- 1000 tons

US $1.00/KG2025-04-21
- CAS:
- 461-58-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt